Table of Contents
List of Tables
- Chapter 2
- Chapter 3
- Chapter 4
- Chapter 7
- Chapter 9
- Chapter 10
- Chapter 12
- Chapter 13
- Chapter 17
- Chapter 19
- Chapter 20
- Chapter 21
- Chapter 22
- Chapter 24
- Chapter 27
List of Illustrations
- Chapter 1
- Chapter 2
- Chapter 3
- Chapter 4
- Chapter 5
- Chapter 6
- Chapter 7
- Chapter 8
- Chapter 9
- Chapter 10
- Chapter 11
- Chapter 12
- Chapter 13
- Chapter 14
- Appendix
- Chapter 1
- Chapter 2
- Chapter 3
- Chapter 4
- Chapter 5
- Chapter 6
- Chapter 7
- Chapter 8
- Chapter 9
- Chapter 10
- Chapter 11
- Chapter 12
- Chapter 13
- Appendix
Guide
Pages
APPENDIX
Structure, Genome Organization, and Infectious Cycles of Viruses Featured in This Book
Adenoviruses
Family Adenoviridae
| Selected Genera | Examples |
|---|
| Mastadenovirus | Human adenovirus type 5 |
| Aviadenovirus | Fowl adenovirus 1 |
Adenoviruses are nonenveloped, double-stranded DNA vi- ruses. Human serotypes are very widespread in the population. Infection by these viruses is often asymptomatic but can result in respiratory disease in children (members of species B and C), conjunctivitis (members of species B and D), and gastroenteritis (species F serotypes 40 and 41). Human adenoviruses 40 and 41 are the second-leading cause (after rotaviruses) of infantile viral diarrhea. Adenoviruses share capsid morphology and linear double-stranded DNA genomes, but the members of the genera differ in size, organization, and coding sequences. The Mastadenovirinae comprise more than 100 adenoviruses of humans and other mammals, including mice, sheep, and dogs, and some are oncogenic in rodents. Study of human adenovirus transformation of cultured cells has provided fundamental information about mechanisms that control progression through the cell cycle and oncogenesis. Characteristic features of the reproduction of these viruses include stereotyped temporal control of viral gene expression and an unusual mechanism of initiation of viral DNA synthesis (protein priming). Mastadenoviral genomes also include genes transcribed by cellular RNA polymerase III.
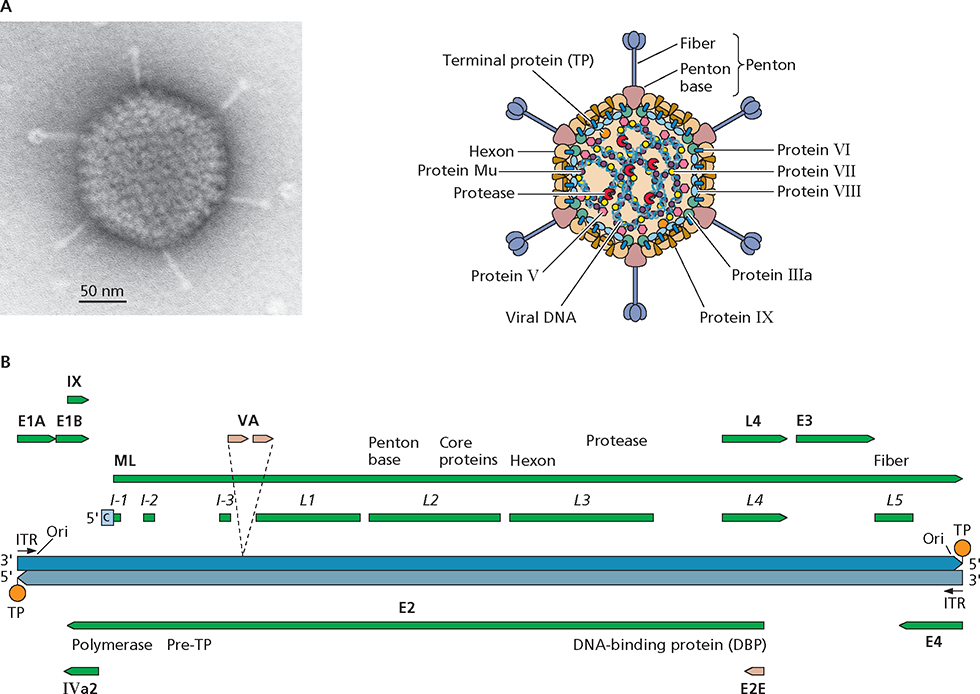
Structure and genome organization of human adenovirus type 5. (A) Virus particle structure. The electron micrograph shows a negatively stained human adenovirus type 5 particle. Courtesy of M. Bisher, Princeton University, Princeton, NJ. (B) Genome organization. The DNA genome length is 36 to 38 kbp. Green and tan arrows represent primary products of RNA polymerase II and III transcription, respectively, and are labeled in bold type. Coding sequences for viral proteins or families of major late mRNAs are also indicated. The coding sequences for viral proteins that perform related functions, such as the viral genome replication proteins encoded within the E2 transcription unit and the L2 core proteins, are often organized together in the viral genome. Hatched lines show splicing of the major late (ML) tripartite leader. ITR, inverted terminal repetition; Ori, origin of replication.
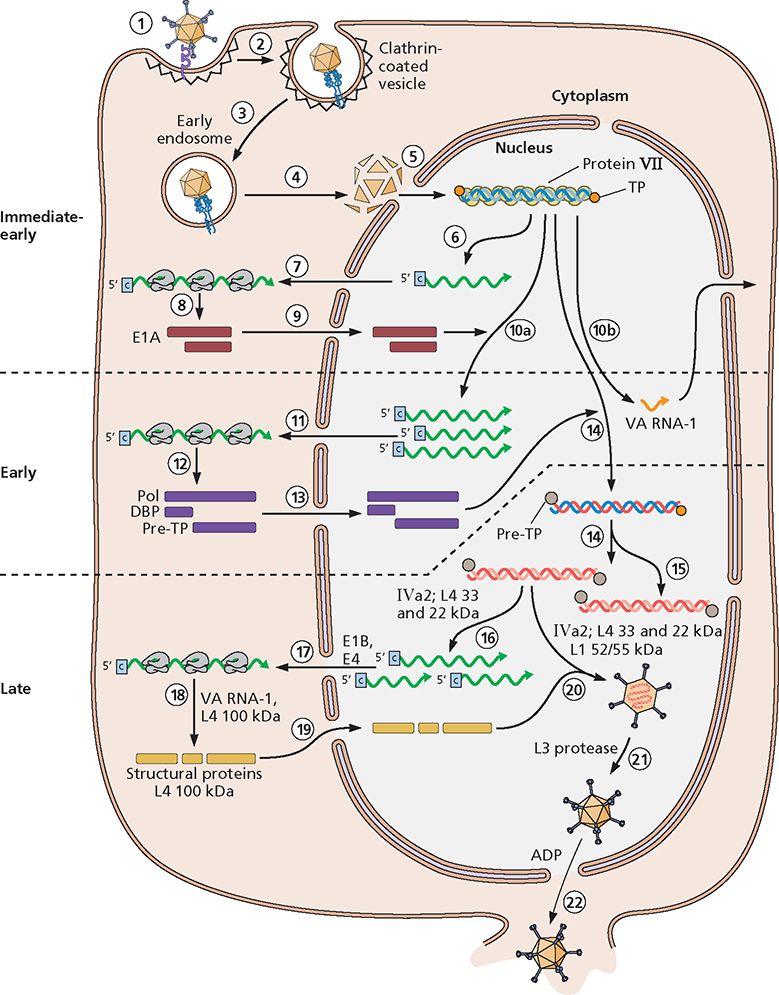
, ML) requires the late IVa2 and L4 proteins. (17) Processed late mRNA species are selectively exported from the nucleus as a result of the action of the E1B 55kDa and E4 Orf6 proteins. (18) Their efficient translation requires the VA RNA-I and the late L4 100-kDa protein. (19) The latter protein also serves as a chaperone for assembly of trimeric hexons as they and the other structural proteins are imported into the nucleus. (20) Within the nucleus, capsids are assembled from these proteins and the progeny viral genomes to form noninfectious immature virus particles. Assembly requires a packaging signal located near the left end of the genome, as well as the IVa2, L1 52/55-kDa, and L4 22- and 33-kDa proteins. Immature particles contain the precursors of the mature forms of several proteins. (21) Mature virus particles are formed when these precursor proteins are cleaved by the viral L3 protease, which is assembled into the core. (22) Progeny virus particles are released, usually upon destruction of the host cell via mechanisms that are not well understood, although the E3 adenovirus death protein (ADP) facilitates exit of particles from the nucleus.
Arenaviruses
Family Arenaviridae
| Selected Genus | Example |
|---|
| Arenavirus | Lymphocytic choriomeningitisvirus |
The Arenaviridae are enveloped viruses and have a bisegmented RNA genome. Their name is derived from the sandy (arenosus; Latin) appearance of viral particles when viewed in the electron microscope. A prototype member of this family, lymphocytic choriomeningitis virus, has been used to elucidate essential principles of the host immune response to viral infection. Arenaviruses cause chronic, usually asymptomatic infections in rodents, the natural host. Contact with infected mice and rats (typically by bite) can result in zoonotic transmission, with outcomes in humans ranging from asymptomatic infection to febrile illness, aseptic meningitis, and often fatal hemorrhagic fevers. Arenaviruses are categorized into Old World and New World serogroups, based on geographical and genetic parameters.
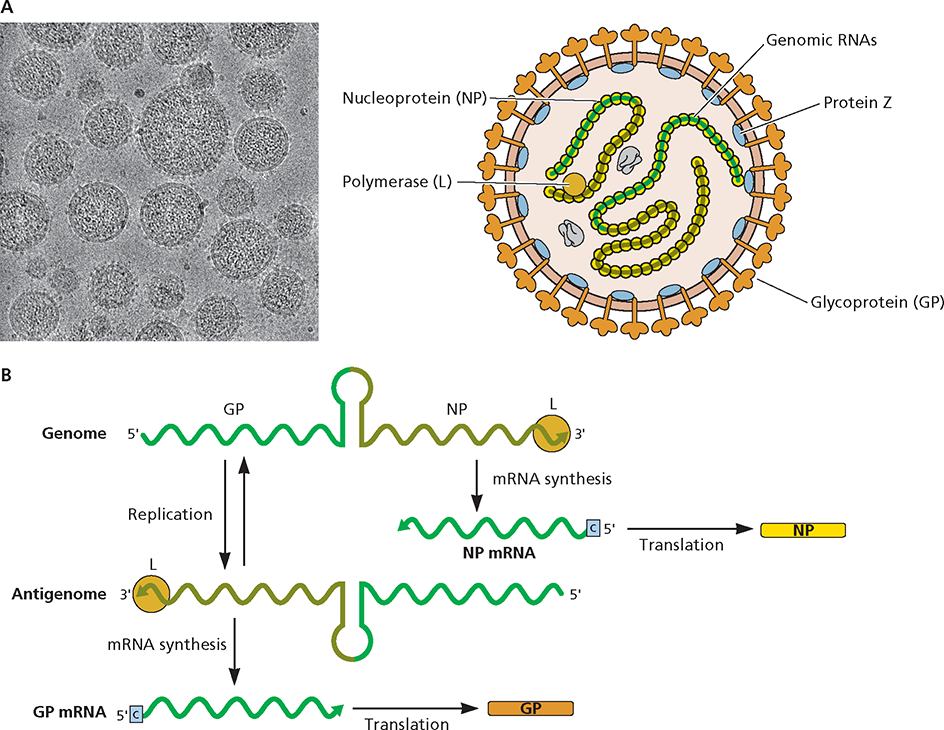
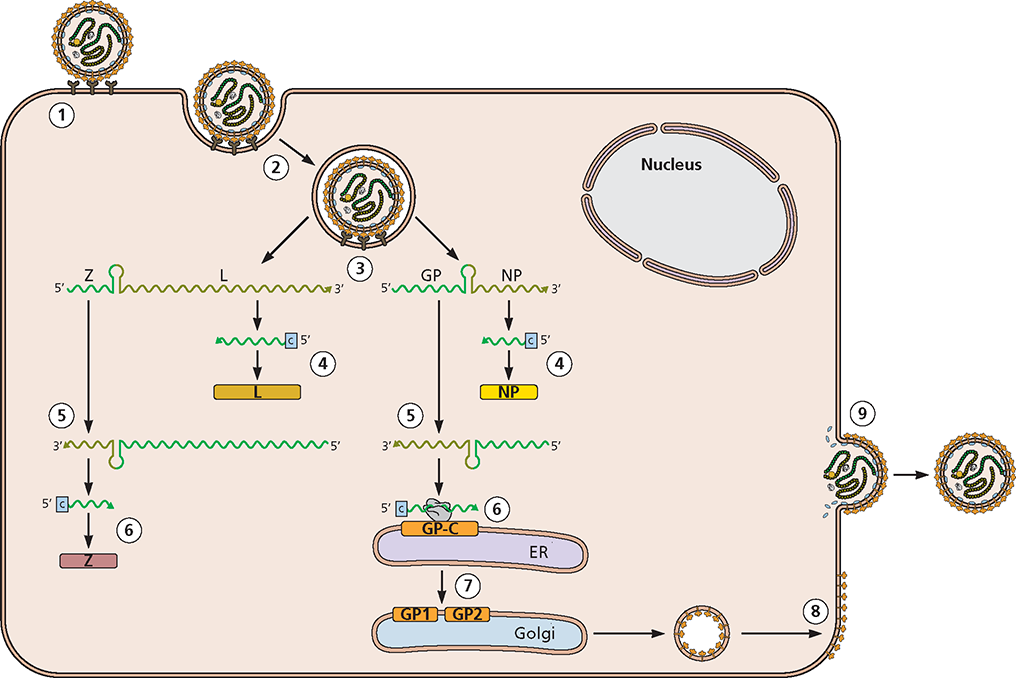
Structure and genome organization. (A) Virus particle structure. Cryo-electron micrograph of particles of the arenavirus lymphocytic choriomeningitis virus. Image courtesy of Michael J. Buchmeier, University of California Irvine and Benjamin W. Neuman, School of Biological Sciences, University of Reading, United Kingdom. (B) Genome organization. The ambisense viral genome comprises two segments: large (L; 7.2 kb) and short (S; 3.5 kb). The L segment encodes the RNA-dependent RNA polymerase (L) and an accessory protein (Z) that functions in genome packaging, particle assembly, and budding. The S segment encodes a surface glycoprotein (GP), which binds to the viral receptor and mediates target cell recognition and entry, and a histone-like nucleocapsid protein (NP) that, with the viral RNAs, forms the ribonucleoprotein. For simplicity, only expression of genes on the S segment is shown, but the same process occurs for the L segment. Upon entry of the viral RNA into the host cell cytoplasm, the viral L protein (shown as an orange ball), which enters the cell with the infecting particle, binds to the 3 end of the RNA and synthesizes the (+) strand NP mRNA, which is then translated. Replication of the genomic RNA into a complementary antigenome allows synthesis of GP mRNA. This mechanism of gene expression results in temporal control of viral gene expression.
) is the template for synthesis of a complementary antigenome by the viral RNA-dependent RNA polymerase (L).