Department of Microbiology, Immunology, and Molecular Genetics, Molecular Biology Institute, University of California, Los Angeles, CA 90095, USA
Copyright
Academic Press is an imprint of Elsevier
225 Wyman Street, Waltham, MA 02451, USA
525 B Street, Suite 1800, San Diego, CA 92101-4495, USA
Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands
The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK
32 Jamestown Road, London NW1 7BY, UK
First edition 2013
Copyright 2013 Elsevier Inc. All rights reserved
No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher
Permissions may be sought directly from Elseviers Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865 843830; fax (+44) (0) 1865 853333; email: , and selecting Obtaining permission to use Elsevier material
Notice
No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made
ISBN: 978-0-12-416749-0
ISSN: 1874-6047
For information on all Academic Press publications visit our website at store.elsevier.com
Printed and bound in USA
13 14 15 16 11 10 9 8 7 6 5 4 3 2 1

Preface
The Ras superfamily G-proteins function as a molecular switch that regulates a variety of biochemical reactions in the cell. These proteins play important roles in cell growth, proliferation, morphology, motility, and others. Activation of these proteins is associated with human cancer and diseases. Over the years, a variety of approaches have been taken to develop small molecule inhibitors of the Ras superfamily G-proteins. This and the next volume (Volumes 33 and 34) provide the first publications of comprehensive overview of these studies.
I am very grateful to the authors for their effort in providing exciting chapters that detail their original approach to the problem. I also thank Mary Ann Zimmerman of Elsevier for her guidance, Helene Kabes of Elsevier for her support, and Phoebe Phan of UCLA for her communication and assistance during the preparation of this volume.
Fuyuhiko Tamanoi
May 2013
Chapter One
The Ras Superfamily G-Proteins
Ashley L. Tetlow and Fuyuhiko Tamanoi
Abstract
The Ras superfamily G-proteins are monomeric proteins of approximately 21 kDa that act as a molecular switch to regulate a variety of cellular processes. The structure of the Ras superfamily G-proteins, their regulators as well as posttranslational modification of these proteins leading to their membrane association have been elucidated. The Ras superfamily G-proteins interact at their effector domains with their downstream effectors via proteinprotein interactions. Mutational activation or overexpression of the Ras superfamily G-proteins has been observed in a number of human cancer cases. Over the years, a variety of approaches to inhibit the Ras superfamily G-proteins have been developed. These different approaches are discussed in this volume.
Keywords
Ras superfamily G-proteins; Ras; Rho; Rab; Arf; Ran; G-motifs; Crystal structure; NMR structure; GEF; GAP; Effectors; Posttranslational modification; Membrane association
1 The Ras Superfamily G-Proteins
The Ras superfamily G-proteins comprise more than 150 monomeric proteins of approximately 21 kDa (].
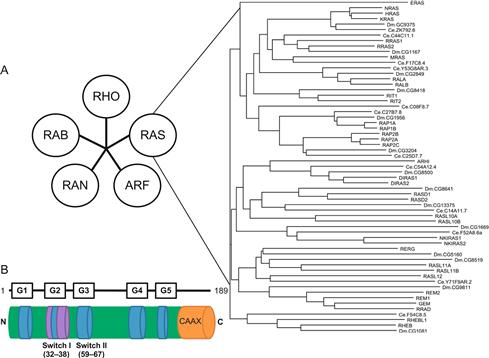
Figure 1.1 ].
This superfamily is divided into subfamilies. While there are overlaps in the cellular processes they affect, general comments can be made concerning processes that each subfamily proteins affect. The Ras subfamily proteins are involved in proliferation, growth, and gene expression and include three isoforms of Ras: K-Ras, N-Ras, and H-Ras [].
Conserved motifs among the Ras superfamily proteins are depicted in ]. In the case of the Ras or Rho family G-proteins, they end with the so-called CAAX motif where C is cysteine, A is an aliphatic amino acid, and X is the C-terminal residue. Rab proteins end with the CC or CXC motif. The C-terminal motifs are recognized by protein prenyltransferases that initiate a series of C-terminal modification events that are critical for their membrane association (discussed below).
2 Overview of Structure, Activity, and Regulatory Proteins
2.1 Three-dimensional structure
Many proteins in the Ras superfamily G-proteins are crystallized and their structures have been elucidated [].
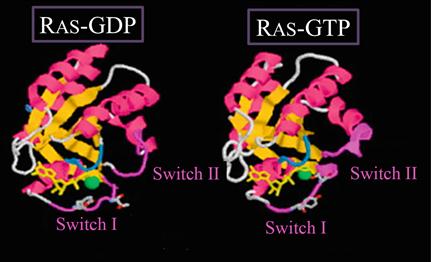
Figure 1.2 ].
Comparison of the GTP-bound form and the GDP-bound form of Ras revealed that there are mainly two regions of the protein that exhibit conformational change []. One of these regions (switch I) is the so-called effector domain where interactions with downstream effector proteins take place. The other region, called switch II, is in the region encompassing residues 5967. This region is involved in the interaction with an activator called GEF (GDP/GTP exchange factor). Thus, structural studies provided mechanistic basis of the action of the Ras superfamily G-proteins.
NMR analyses of the Ras superfamily G-proteins have also been carried out. Solution structure of full-length farnesylated and nonfarnesylated H-ras proteins has been elucidated [].
2.2 GEF and GAP regulate the activities of the Ras superfamily G-proteins
The Ras superfamily G-proteins have intrinsic activities that are stimulated by regulatory proteins as shown in ].
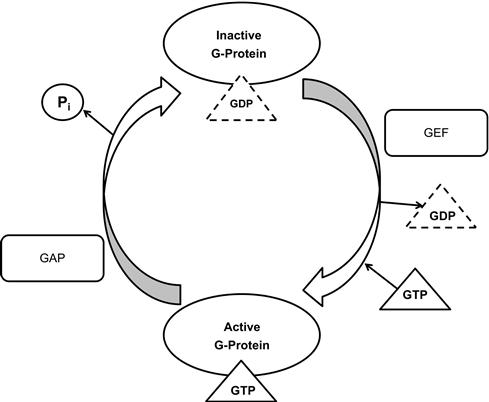
Figure 1.3 Schematic model of the molecular switch mechanism of Ras superfamily class GTPases in continuous cycle between active and inactive states. The proteins GAP and GEF aid in the transition of GTPases. GEF (guanine nucleotide exchange factors) removes GDP from inactive G-proteins. GAP (GTPase activating protein) hydrolizes GTP and an inorganic phosphate is released.
The bound GDP can be exchanged with GTP, but this intrinsic exchange activity is very slow. This is stimulated by a group of regulatory proteins called GEF. GEF interacts with GDP-bound form and exchanges bound GDP with fresh GTP. The Cdc25 family acts on Ras, Ral, and Rap and includes Cdc25, SOS, and EPAC. The crystal structure of SOS/Ras complex has been elucidated [].












