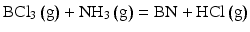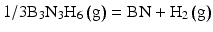Introduction
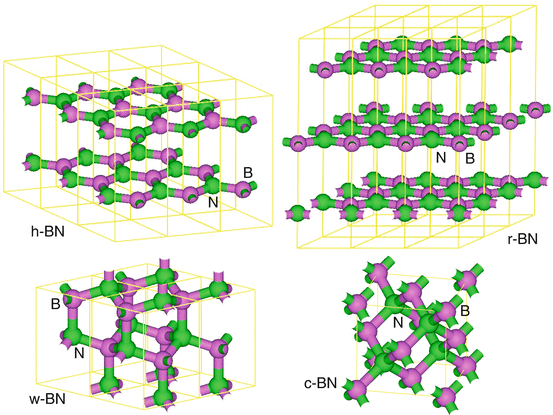
Boron nitride (BN) has very similar structures to carbon (C) materials such as graphite, diamond, fullerene, nanotube, and amorphous structures. The BN has more superior properties of high oxidation resistance [.
Fig. 1
Structure models of hexagonal BN, rhombohedral BN, wurzite BN, and cubic BN
The present chapter displays BN nanoparticle materials and nanocapsules, which were synthesized by thermal annealing, chemical vapor deposition, and arc melting. Structures of the BN nanomaterials were characterized by using X-ray diffraction, transmission electron microscopy (TEM), electron diffraction, and high-resolution electron microscopy (HREM) [], and the properties were also investigated and presented. To confirm and investigate their atomic structures, electronic states, stabilities, and hydrogen gas storage, energy calculations were performed by using molecular orbital and molecular mechanics calculations. The present studies serve as a guide for design and synthesis of BN nanoparticle and nanocapsule materials, and they are expected as future nanoscopic-scale devices.
Formation of BN Nanoparticles by Chemical Vapor Deposition
Boron nitride synthesized by chemical vapor deposition (CVD-BN) has been used in various practical fields as crucibles for semiconductor materials, high-temperature jigs, and insulators, utilizing its high purity, high density, and chemical inertness. The effects of deposition temperature and total gas pressure on the crystal structure, density, and microstructure of CVD-BN had been studied [].
The purpose of the present work is to investigate the formation of BN nanoparticles in CVD-BN by TEM , electron diffraction, and molecular orbital calculations. TEM, HREM, and electron diffraction studies were carried out for structural analysis, since they are very important methods for atomic structure analysis []. The effects of deposition temperature, total gas pressure, and the synthesis gas on the structures of BN nanoparticles were also investigated. The present work will give us guidance for the synthesis of CVD-BN with nanostructures. BN nanoparticles were produced from BCl3NH3H2 and B3N3H6 systems at gas pressures of 0.2 ~ 30 Torr and temperatures of 1,400 ~ 2,100 C on the graphite substrates.
Figure ].
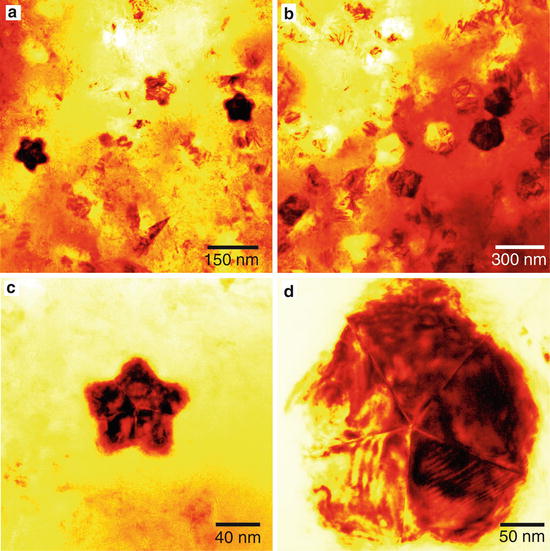
Fig. 2
Transmission electron micrographs of boron nitride nanoparticles produced from BCl3NH3H2 system at ( a ) 1,800 and ( b ) 2,000 C and a total gas pressure of 5 Torr. ( c ) and ( d ): enlarged images of nanoparticles
A TEM image of CVD-BN produced from B3N3H6 gas system at 1 Torr and 2,000 C is shown in Fig. , the 002, 102, and 112 reflections are very weak, which indicates that the c-axis of the h-BN is almost perpendicular to the CVD-BN plate.
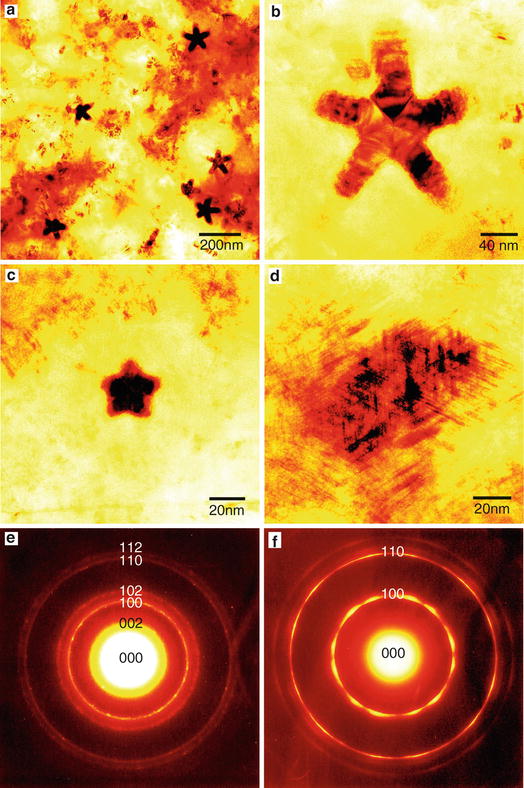
Fig. 3
Transmission electron micrographs of boron nitride nanoparticles produced from B3N3H6 at various deposition temperatures and a total gas pressure of 1 Torr. ( a ) and ( b ) 2,000, ( c ) 1,800, and ( d ) 1,600 C. Electron diffraction patterns of CVD-BN synthesized at deposition temperatures of ( e ) 2,000 and ( f ) 1,600 C and a total gas pressure of 1 Torr
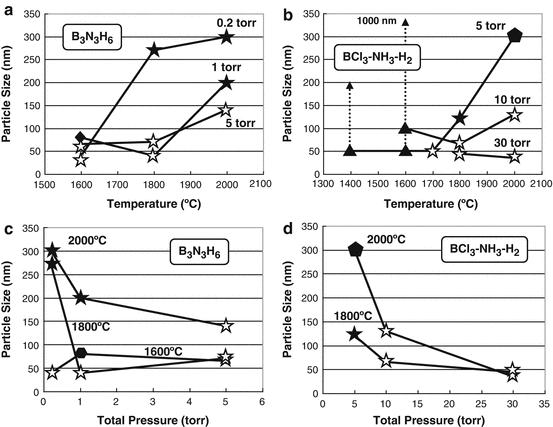
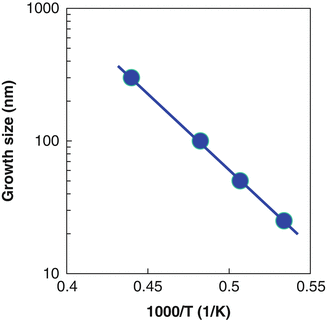
Size dependences of BN nanoparticles on the deposition temperatures of CVD-BN produced from BCl3NH3H2 and B3N3H6 systems are shown in Fig. show size dependences of BN nanoparticles on the gas pressures produced from BCl3NH3H2 and B3N3H6 systems, respectively. Sizes of nanoparticle were decreased by the increase of pressures. It is considered that low pressure and high temperature affect the formation of BN nanoparticles with fivefold symmetry, which might be due to the growth speed of the boron nitride on graphite substrates.
Fig. 4
( a , b ) Size dependences of boron nitride nanoparticles produced from B3N3H6 and BCl3NH3H2, respectively, on the deposited temperatures. ( c , d ) Size dependences of boron nitride nanoparticles produced from B3N3H6 and BCl3NH3H2, respectively, on the gas pressures. : pentagonal-shaped particle, : fivefold star-shaped particle, : a few star-shaped particle, : hexagonal-shaped particle, : rhombohedral boron nitride, : hexagonal boron nitride
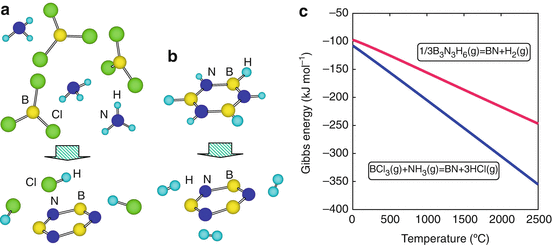
Schematic illustrations of chemical vapor deposition reaction for the BCl3NH3H2 and B3N3H gas systems are shown in Fig.. Reactions of BN formation at 2,000 C are as follows:
Fig. 5
Schematic illustration of CVD reaction for ( a ) BCl3NH3H2 and ( b ) B3N3H6 gas system. ( c ) Calculated Gibbs energies for BN formation
Gibbs free energy (G) for these reactions was calculated as 305 kJ mol1 and 217 kJ mol1 for the formulas Eqs. ].
Fig. 6
Growth size of fivefold BN nanoparticles at temperatures ranging from 1,600 C to 2,000 C