The main factors leading to skin cancer are pale skin Fitzpatrick skin types I and II > III and IV [].
1.1 Actinic Keratoses and Squamous Cell Carcinoma
Repeated UVR exposure of the skin in man leads to clones of cells accumulating which contain mutated p53. These cells contain p53 functioning in its mutated form as a tumour promoter. With ongoing UV exposure, the p53 patches become more numerous [].
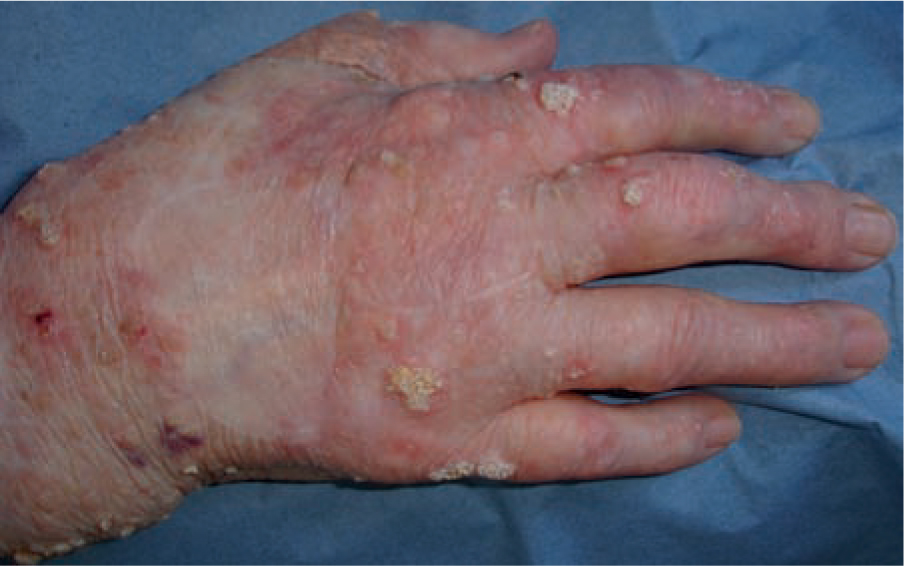
Actinic keratoses first present on sites of maximum UVR exposure and along with solar elastosis are the first objective clinical evidence of cellular dysplasia within the epidermis. Actinic keratoses, histologically, are seen as partial thickness dysplasia, usually the lower third of the epidermis. Progression to full thickness dysplasia may occur and an estimated one in 50 AKs progress to squamous cell carcinoma (SCC); the true rate of progression of AKs to SCC in any one person is unknown. Squamous cell carcinoma in situ is full thickness dysplasia and is a very common skin cancer of the elderly. It is not recorded in many national cancer registers and many lesions are treated topically, so the true incidence is not documented. Frequently multi-focal squamous cell carcinoma is associated with the presence of human papilloma virus (HPV) and is especially common in immunosuppressed individuals []. Frequently, plane warts, multi-focal Bowen's disease and HPV infection are seen on the lower legs of women with considerable UV exposure and fair skin type. Nowadays, the multi-focal Bowen's disease is less common which used to be a consequence of previous arsenic exposure which was prevalent in iron tonics in Europe until the 1950s. which lead to the various complementation groups of xeroderma pigmentosum or defects of post replication DNA repair (XP variant). Such enzyme-dependent DNA repair is error prone, even with normal enzyme function and vestiges of DNA damage may remain.
DNA damage induced by UVR often occurs in crucial genes such as the p53 gene and other tumour suppressing genes; if this damage is not repaired, the p53 gene function is altered from the normal function of the gene (a tumour suppressor gene) to that of a tumour-promoting gene []. In response to incident UV on the skin which leads to DNA lesions, the function of p53 is to halt the cell in S phase, permitting repair of such DNA damage. If the cell has too much DNA damage to be repaired, the p53 gene triggers a series of events through the caspase pathway which culminates in cell suicide (so-called programmed cell death or apoptosis), a non-inflammatory, harmless way of eliminating cells which are beyond repair. Apoptosis therefore is an error-free method of removing cells with significant UV-induced DNA damage. In the Li Fraumeni syndrome, no p53 is produced; patients with such syndrome are prone to multiple cancers including malignant melanoma. In knock-out mice with no p53
Disseminated superficial actinic porokeratosis is a peculiar, often genetically influenced, clonal expansion of epidermal cells leading to UV-distributed subtle annular lesions which may occasionally be pre- malignant. More frequently porokeratosis of Mibelli, a much larger variant, leads to squamous cell carcinoma. Poroke-ratosis is seen more frequently in immunosuppressed individuals.
Squamous cell carcinoma is directly related to total UVR dose. The larger the dose of UVR, the paler the colour of the skin: the earlier the onset of SCC. Actinic keratoses occur in childhood in XP. In this genetic disease, failure of the DNA repair mechanism leads to retention of UV-induced DNA lesions and, as a consequence, accelerated photo-ageing and pre-malignant lesions occur together with malignant skin cancers at a rate 1,000-fold greater than the general public. Thus, from this collection of diseases, we understand the importance of the DNA repair complex. Skin cancer may develop in early adult life in skin type I or albinism in equatorial/tropical regions where exposure of a skin without the ability to absorb incident UVR is particularly detrimental. Eumelanin (which is black melanin) has the ability to absorb UVR without augmenting its effects. Phaeomelanin (red-yellow melanin) found in skin type I and II in relatively greater amounts appears to act as a photosensitiser, actually generating free radicals and augmenting the effects of UVR exposure. In albinism in sub-Saharan Africa, skin without the ability to pigment is overwhelmed by UVR and readily develops basal and particularly squamous cell carcinoma in a multi-focal distribution. The other group of patients who develop very large numbers of skin cancers are the long-term immunosuppressed individuals, specifically those with pale skin, with onset of skin cancer and 2030 years earlier than equally exposed normal adults even in high latitude countries. Thus, within all these diseases, the importance of DNA repair, pale skin and immunosuppression are highlighted dramatically as important components of photoprotective mechanisms in human skin.