Introduction
The process of blood vessel growth, generally referred to as angiogenesis, is pivotal for the development, homeostasis and function of all tissues and organs in the organism, but also for progression of most serious diseases including ophthalmic disorders, cancer, cardiovascular disorders, inflammatory disorders and chronic skin ulcers []. It is now clear that the regulation of tissue/organ development, physiology and disease progression by blood vessels is highly complex and context-dependent, but certain general concepts and factors are central and important for angiogenesis during development and in most diseases. Such general concepts will be the focus of this chapter.
The concept of blood vessel formation and growth was first mentioned by the Greek philosopher Aristotle in the fourth century BC [], but such combination therapies remains to be thoroughly tested in clinical studies.
In many cases angiogenic factors may act in a tissue-dependent manner. I.e. the factors that is important for growth of blood vessels in the brain or eye, such as Wnt7a/b, Grp124 [].
In this chapter we aim to give an overview of the key features of blood vessels, their structure and function and how blood vessels are involved in regulation of tissue functions. In the second part we will focus particularly on the mechanisms regulating vessel growth, maturation and regression, and discuss key differences between the growth and function of healthy versus pathological vessels. Finally we will introduce the concept of lymphangiogenesis and how lymph vessels develop, function and may contribute to disease under pathological conditions.
Blood Vessel Physiology and Function
The first and foremost function of blood vessels is to supply oxygen and nutrients (mainly glucose and lipids) to support cellular metabolism. A large fraction of the blood is therefore dedicated to transport of oxygen via hemoglobin-containing erythrocytes that are responsible for approximately 50% of the blood volume. In the cell-free fraction, the most abundant proteins including albumin and lipoproteins are dedicated to transport of water in-soluble lipids/amphiphiles, and the plasma has a high buffer capacity as a way to combat acidosis or reactive oxygen species that are unavoidable side-effects from oxidative cellular metabolism. The abundance of these oxygen- or lipid-binding factors in the blood also reflect the facts that free oxygen is among the most reactive compounds in the organism and because free lipid is detrimental to vascular physiology. Blood is delivered through the vasculature to all metabolically active tissues and collected by veins which bring waste products, primarily carbon dioxide (CO2), urea and other metabolites through the kidneys and the liver to the lungs, where the last of the waste products (CO2) is released and the blood is reoxygenated. This constant circulation of blood is necessary for its function blood that accumulate in tissues due to insufficient venous drainage, persistent circulation in futile loops or hemorrhage rapidly loose the tissue-supportive function and rather become a reservoir for tissue-damaging factors including cytotoxic cells and compounds []. As such, blood is only beneficial to tissues if it is also effectively collected and recycled.
The metabolic activity of a tissue is the primary regulator of blood perfusion. When metabolic activity increase this will lead to tissue hypoxia, which is the most important physiological signal for increasing perfusion. This is perhaps most clearly exemplified in the brain where the activity of different brain regions is directly coupled to changes in perfusion; all techniques used to measure brain activity today actually measure blood perfusion and not the activity of the nerves or other brain cells themselves [).
Fig. 1.1
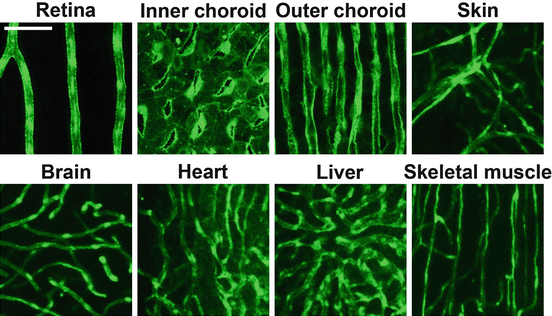
Morphological differences between capillary beds of various tissues. Confocal micrographs of blood vessels, shown in green , found in the retina, inner and outer choroid, skin, brain, heart, liver and skeletal muscle of adult transgenic Tg(fli:EGFP)y1 zebrafish, expressing enhanced green fluorescent protein (EGFP) in endothelial cells. The white size bar indicate 100 m
Fig. 1.2
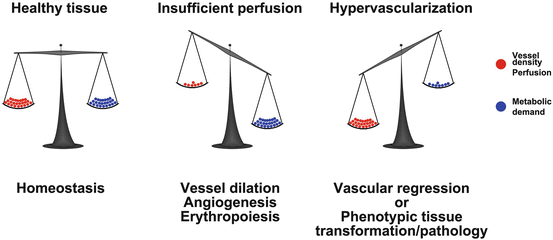
Vascular endowment is balanced by the metabolic needs of the tissue. In healthy tissue the vessel density and perfusion ( red balls ) is balanced according to the metabolic activity of the tissue ( blue balls ; left image ). Should the metabolism increase ( middle image ), perfusion and oxygen delivery will also increase through vessel dilation, angiogenesis and erythropoiesis. Is however the vascular density or perfusion higher than the needs of the tissue ( right image ), excessive vessels will regress or the metabolic needs of the tissue will increase (phenotypic switch). In diseased tissues, excessive vessels do not regress but rather sustain pathological transformation or growth of the tissue
Blood vessels, however, are not only a conduit for transport of oxygen, nutrients and metabolic waste products. Many factors important for organizing the functions of various organs to act in concert with each other (i.e. endocrine regulators) also use the vasculature as a route of communication and the vessels play an active role in the signaling process. For example, insulin-dependent and in-dependent glucose uptake by cells in the brain, where there is no passive leakage of glucose across the capillary, is mediated by active transport of glucose across the wall of the blood vessels by Glut1 and Glut4 glucose transporters [].
The vasculature not only transports chemicals and cells, it is also critical for the thermoregulation by transporting heat [], although many of the other tissues exhibit similar metabolic activity.
Locally, in the tissues, blood vessels also provide key signals for the surrounding cells which are crucial for the specific function. Especially un-differentiated stem- and progenitor cells are known to exist predominantly in so-called vascular nieches, in which these cells make direct contact to endothelial cells, contacts that are crucial for maintaining the undifferentiated state of the cells [] indicating that vessel contact may induce or maintain processes required for alternative differentiation and associated functions of macrophages.
During development, the vasculature provide key signals required for organogenesis, regulation of organ size and shape as well as differentiation and specification of cell types in the various organs. The development of the alveoli in the lungs, for example, depends on signals provided by the developing lung vasculature [] indicating that there may be some still poorly characterized signals being produced by the vessels which are important for organ development.