chapter
VST.Epub.contentReady(window, document); VST.cfiBase='/4';
Integrated Imaging Modalities in the Cardiac Catheterization Laboratory
MICHAEL S. KIM, MD, and ROBERT A. QUAIFE, MD
INTRODUCTION
Over the last decade, there has been an exponential growth in the number of transcatheter therapies designed to treat both congenital and acquired structural heart disease (SHD) pathologies. Along with this growth have come major advances in image guidance including three-dimensional transesophageal echocardiography (3D TEE), cardiac computed tomographic angiography (CCTA), and magnetic resonance imaging and angiography (MRI/MRA). In contemporary practice, catheter-based treatments of various structural heart and valve diseases have become increasingly reliant on accurate preprocedural imaging assessment and intraprocedural guidance to maximize outcomes and minimize complications.
A major challenge facing all SHD interventionalists and imaging specialists, however, centers on the importance of integrating efficiently multiple imaging modalities so as to prevent sensory imaging overload. Oftentimes, many operators also struggle with mentally translating two-dimensional (2D) imaging sequences (eg, CCTA, 2D echocardiography) into accurate and useful 3D spatial images in their own minds to both effectively preplan and efficiently perform complex SHD procedures. To overcome these barriers, imaging manufacturers are actively developing new software tools that are designed to take the complexities of multimodality imaging integration out of the hands of the operators, while simultaneously giving back to the operator a simplified and efficient mechanism by which to manipulate and analyze the processed images.
This chapter, through several clinical examples, will highlight how both high-quality preprocedure imaging and intraprocedural imaging using novel multimodality image integration tools can be effectively used to guide complex SHD interventions.
CASE 1Right Ventricular to Left Atrium Fistula Repair
A 55-year-old male with a history of an endocardial cushion defect that was surgically repaired at age 7 years with a patch at the septum primum and inlet ventricular septal defect (VSD) was referred to evaluate and treat a residual right ventricular (RV) to left atrial (LA) fistula. He had a recent biventricular pacemaker/internal cardiac defibrillator (ICD) placed for asymptomatic complete heart block in the setting of left ventricular (LV) dysfunction. After device implantation, he began complaining of new visual symptoms (intermittent vision loss in his left eye) concerning for transient ischemic attacks (TIAs); a brain MRI could not be obtained owing the presence of ). Given the concern that the patient would be at risk for forming small thrombi on his ICD leads that both may have and could in the future embolize paradoxically, the decision was made to proceed with transcatheter closure of the residual fistula.

Video 1-1
As part of his preprocedure evaluation, the patient underwent a CCTA to better elucidate the size and location of the fistula (). Postprocedure TEE and TTE demonstrated no residual flow across the device. The patient was discharged on postprocedure day 1 and remains in good condition.

Video 1-2
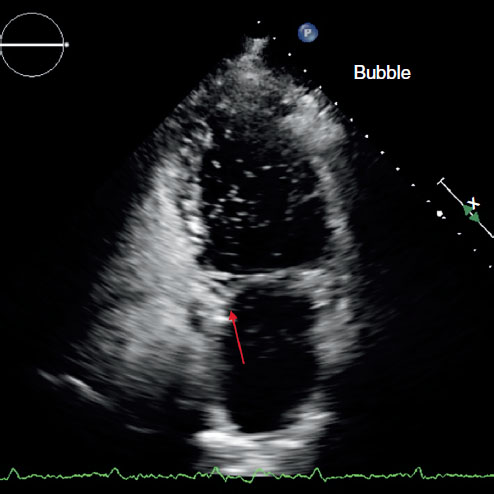 FIGURE 1.1
FIGURE 1.1 TTE agitated saline contrast (bubble) study through a peripheral vein demonstrating a communication between the RV and LA (arrow) with right to left shunting.
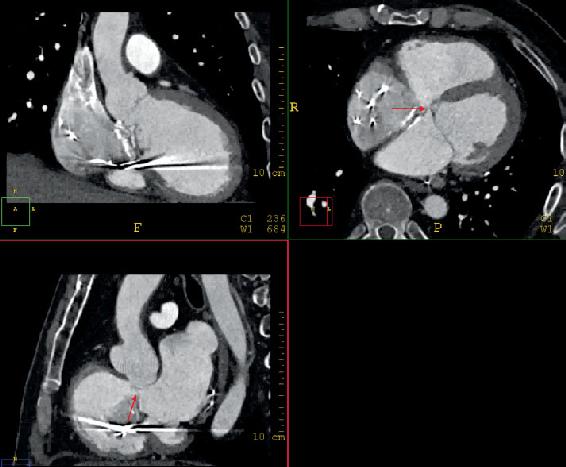 FIGURE 1.2
FIGURE 1.2 Multiplanar re
construction of the CCTA demonstrating the fistula between the RV and LA (arrows) in orthogonal planes. The fistula was dynamic in nature and measured approximately 7 mm in diameter and 6 mm in length during ventricular systole.
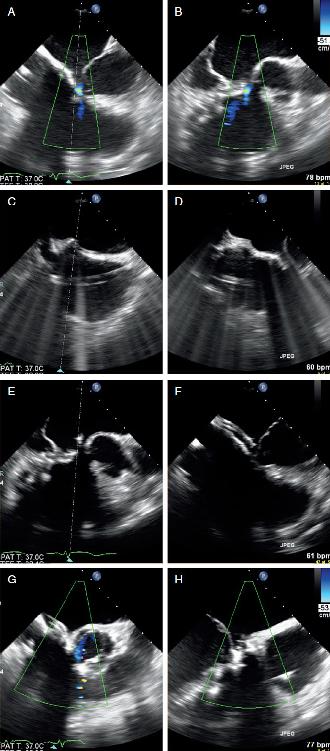 FIGURE 1.3
FIGURE 1.3 Intraprocedure TEE.
A and B, 4 chamber and LV outflow tract view showing color flow between the LA and RV.
C and D, Location of the transseptal puncture inferior (C) and posterior (D).
E and F, Orientation of the steerable guide catheter directly into the location of the fistula from the LA.
G and H, Occluder device across the fistula with absence of flow by color Doppler indicating complete closure.
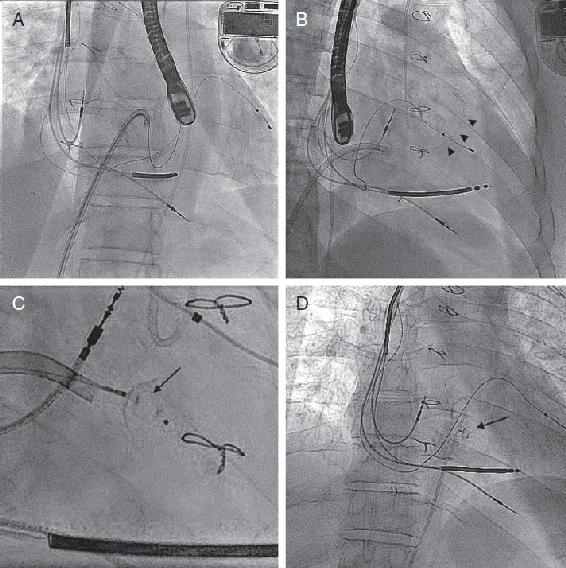 FIGURE 1.4
FIGURE 1.4 Fluoroscopic images of RV to LA fistula closure.
A, With the steerable guide catheter (arrowheads, positioned guide catheter) pointed into the LA side of the fistula, a Magic Torque wire is advanced across the defect and out the LV outflow tract across the aortic valve into the ascending aorta (Ao).
B, A 5 French diagnostic catheter is advanced over the wire into the ascending Ao, and the wire is removed.
C, Amplatzer Duct Occluder II device (arrow, showing deployed device) is fully deployed across the fistula.
D, Final angiography demonstrating stable placement of the occluder device.
CASE 2Prosthetic Mitral Paravalvular Leak Repair
A 70-year-old female with a history of rheumatic heart disease underwent surgical mitral valve replacement with a 29 mm porcine bioprosthesis. Although her immediate postoperative course was uneventful, she presented several weeks after surgery with decompensated heart failure symptoms (New York Heart Association Class III-IV). A TTE and TEE confirmed the presence of a severe paravalvular leak located on the posterior aspect of the sewing ring. There was also evidence of mild hemolysis. A cardiothoracic surgeon was consulted who felt that a reoperation would put the patient at excessive risk given her current clinical state, and thus she was referred for transcatheter paravalvular leak (PVL) repair.
The patient underwent a preprocedure CCTA to evaluate the size and extent of the posterior PVL as well as assess for any additional defects ( ). The patient was discharged on postprocedure day 2 and remains in excellent clinical condition with NYHA Class I symptoms and no evidence of hemolysis.

Video 1-3

Video 1-4
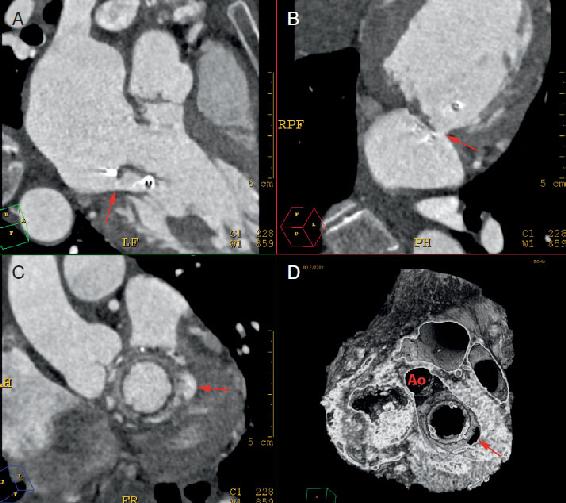 FIGURE 1.5
FIGURE 1.5 CCTA of posterior mitral paravalvular leak.
A-C, 2D multiplanar reconstruction of the mitral annulus demonstrating a large paravalvular leak located on the posterior aspect of the sewing ring (arrows).









 FIGURE 1.1 TTE agitated saline contrast (bubble) study through a peripheral vein demonstrating a communication between the RV and LA (arrow) with right to left shunting.
FIGURE 1.1 TTE agitated saline contrast (bubble) study through a peripheral vein demonstrating a communication between the RV and LA (arrow) with right to left shunting. FIGURE 1.2 Multiplanar re
FIGURE 1.2 Multiplanar re FIGURE 1.3 Intraprocedure TEE. A and B, 4 chamber and LV outflow tract view showing color flow between the LA and RV. C and D, Location of the transseptal puncture inferior (C) and posterior (D). E and F, Orientation of the steerable guide catheter directly into the location of the fistula from the LA. G and H, Occluder device across the fistula with absence of flow by color Doppler indicating complete closure.
FIGURE 1.3 Intraprocedure TEE. A and B, 4 chamber and LV outflow tract view showing color flow between the LA and RV. C and D, Location of the transseptal puncture inferior (C) and posterior (D). E and F, Orientation of the steerable guide catheter directly into the location of the fistula from the LA. G and H, Occluder device across the fistula with absence of flow by color Doppler indicating complete closure. FIGURE 1.4 Fluoroscopic images of RV to LA fistula closure. A, With the steerable guide catheter (arrowheads, positioned guide catheter) pointed into the LA side of the fistula, a Magic Torque wire is advanced across the defect and out the LV outflow tract across the aortic valve into the ascending aorta (Ao). B, A 5 French diagnostic catheter is advanced over the wire into the ascending Ao, and the wire is removed. C, Amplatzer Duct Occluder II device (arrow, showing deployed device) is fully deployed across the fistula. D, Final angiography demonstrating stable placement of the occluder device.
FIGURE 1.4 Fluoroscopic images of RV to LA fistula closure. A, With the steerable guide catheter (arrowheads, positioned guide catheter) pointed into the LA side of the fistula, a Magic Torque wire is advanced across the defect and out the LV outflow tract across the aortic valve into the ascending aorta (Ao). B, A 5 French diagnostic catheter is advanced over the wire into the ascending Ao, and the wire is removed. C, Amplatzer Duct Occluder II device (arrow, showing deployed device) is fully deployed across the fistula. D, Final angiography demonstrating stable placement of the occluder device. FIGURE 1.5 CCTA of posterior mitral paravalvular leak. A-C, 2D multiplanar reconstruction of the mitral annulus demonstrating a large paravalvular leak located on the posterior aspect of the sewing ring (arrows).
FIGURE 1.5 CCTA of posterior mitral paravalvular leak. A-C, 2D multiplanar reconstruction of the mitral annulus demonstrating a large paravalvular leak located on the posterior aspect of the sewing ring (arrows).