1.1 Brief Historical Introduction
The initial proof of the existence of very large organic molecules was supplied by Raoult [], who carried out cryoscopic molecular weight determinations on rubber, starch, and cellulose nitrate. By the methods developed by Raoult and by vant Hoff and by the formulation of solution laws, molecular weights of 10,00040,000 were demonstrated. Unfortunately, chemists of that day failed to appreciate this evidence and refused to accept it. The main reason for such a response was the inability to distinguish macromolecules from colloidal substances that could be obtained in low molecular weights. The opinion of the majority of that day was that Raouls solution does not apply to materials in colloidal state.
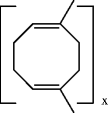
During the period 18901910, the idea of molecular complexes was generally accepted []. It was used to explain polymeric structures in terms of physical aggregates of small molecules. In fact, molecular association was considered polymerization. Thus rubber, for example, was assumed to be composed of short sequences of isoprene units, either as chains or as cyclic structures. The structure of isoprene itself was known, because it was isolated from natural rubber, in 1860. What added to the general confusion was the fact that no one was able to show the existence of end groups in the macromolecules studied. This enhanced the idea that rubber is a ring-like structure, a dimethyl cycloocatadiene. Large numbers of such rings were assumed to be held together by associations, giving rise to colloidal materials. This can be illustrated as follows:
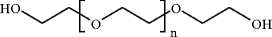
Early synthetic polymeric products were usually discarded as being oils or tars (gooks), and considered as useless. To be fair to the chemists of that period, however, one should not forget that in spite of the general attitude of the time, the structures of some polymers, like polyethylene glycol ( n =6), for instance,
was correctly assigned in 1860, and the concept of extending the structure to very large molecular weights by continued condensation was understood [].
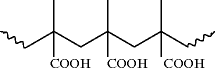
At approximately the same time, poly(methacrylic acid), which we now know to be a linear molecule
was prepared in 1880 [].
One should also acknowledge the fact that in spite of ignorance of structure, many inventors developed ways to convert cellulose into cellulose acetate and then to use the products to form fibers, films, and coatings. Cellulose was also converted to cellulose nitrate and was used to prepare explosives and other products. At the turn of the century, Baekeland formed a hard resin by condensing phenol with formaldehyde [],
The evolvement of our present-day understanding of polymeric structures occurred in the early 1920s. Thus, Staudinger et al. firmly established the existence of macromolecules [].
Now, we know that a typical molecule such as polyethylene can have a contour length of 25,000 , but a diameter of only 4.9 . Such a molecule can be compared in dimensions to a long, snarled clothesline, 75 ft long and 1 in. in diameter. Furthermore, work with naturally occurring macromolecules, such as nucleic acids, for instance, revealed even more startling dimensions. When molecules of virus dinucleic acids were tritium-labeled (whose nuclear emission is less than 1 m) and then autoradiographs prepared, these showed molecules that were about 50 m long []. Such length would signify a molecular weight of 100 million. Similar work carried out on dinucleic acids of bacteria revealed molecular weights of approximately 200 million.
The above figures are, of course, extremes in molecular dimensions. Typical synthetic polymers will range in molecular weights anywhere from ten to several hundred thousand, although synthetic polymers in molecular weight ranges of several million are well known and some are used commercially. Interestingly enough, many of these polymers are prepared through the use of organic reactions that have been known for a long time. Also, new reactions and catalysts are still being discovered and applied to polymer syntheses. It is probably safe to predict that this situation will undoubtedly continue into the distant future.
1.2 Definitions
The word polymer is commonly understood to mean a large molecule composed of repeating units, or mers (from the Greek word meros part), connected by covalent bonds. Such units may be connected in a variety of ways. The simplest is a linear polymer, or a polymer in which the units are connected to each other in a linear sequence, like beads on a string. Many examples of such linear polymers are possible, as, for instance, linear polyethylene:
The terminal units in such molecules must be different from the internal ones to satisfy valence requirements. Polyethylene, like all other polymers, can be written to show the number of repeat units, [CH2CH2] n , by using a number or a letter, like in this case n . It represents the average quantity of mers present in the polymer and is called the degree of polymerization or DP , or the average number of repeat units in the polymeric chain. Thus the average molecular weight of polystyrene with a DP of 100 is 104100 or 10,400. (There are actually several ways of expressing the average molecular weights of polymers. This is discussed further in this chapter).
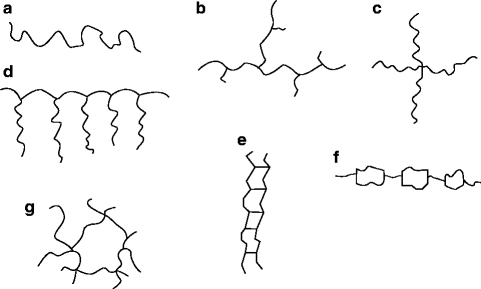
An alternative to a linear polymer is a branched one. The branches can be long or short. Low-density polyethylene, for instance, can have both short and long branches. Linear and branched molecules are shown in Fig. f).
Fig. 1.1
Shapes of polymer molecules. ( a ) Linear polymer, ( b ) branched polymer, ( c ) star-shaped polymer, ( d ) comb shaped polymer, ( e ) ladder polymer, ( f ) semiladder polymer, and ( g ) network structure
When branches of different polymers become interconnected, network structures form. Planar networks resemble the structure of graphite. Three-dimensional networks, or space networks, however, can be compared with diamonds. A network polymer is shown in Fig. g.