1.1 IntroductionWhat Are Inorganic Nanosheets?
Inorganic nanosheets are inorganic plate-like particles with thickness at the nm-level and lateral length in the range of several to thousands of times greater than their thickness. The term nanosheets can essentially be applied to any particle with thickness in the range of a 1 nm to sub-m, and a wide range of materials from graphene to plate-like polymers can satisfy this requirement. However, the term inorganic nanosheets is generally reserved for thin layers or monolayers, with thickness less than several nanometers, obtained by exfoliation of inorganic layered crystals (Fig. ). In this book, we consider inorganic nanosheets in this narrow sense.
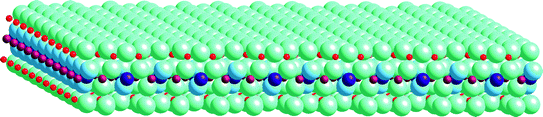
Fig. 1.1
Inorganic nanosheet obtained by exfoliation of montmorillonite (layered clay mineral), indicating the 2:1-type single layer of the clay mineral. Red Si4+, brown Al3+, purple Mg2+, blue-green O2 or OH
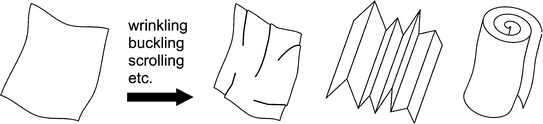
Exfoliated inorganic nanosheets obtained from inorganic layered crystals have characteristics distinguishable from those of typical inorganic particles. Nanosheets are ideally monolayers, or a few layers, of a mother layered crystal. Monolayers are yielded by complete exfoliation of the mother crystals to achieve highly anisotropic layers with thickness around 1 nm. Significance of the monolayer nanosheets is their ultimate thinness as inorganic crystallites. In other words, nanosheets are the smallest units of inorganic crystals that can be manipulated, in analogy with monomolecular layers of organic solids. The thinness of inorganic nanosheets also endows macroscopic softness as nanosheets undergo wrinkling, buckling, and scrolling (Fig. ). Most exfoliated nanosheets retain crystallinity that reflects the crystalline structure of the mother compounds. This contrasts with conventional inorganic nanosheets that are often amorphous or poorly crystalline. These morphological features of the inorganic nanosheets facilitate construction of macroscopic structures by manipulation at the nanoscale and have stimulated development of a wide range of new materials.
Fig. 1.2
Schematic representation of the possible deformations of a nanosheet
The broad range of layered materials that can be exfoliated gives us a rich library of inorganic nanosheets. These materials span elementary substances, such as carbon and phosphorus, to oxides and chalcogenides; inorganic compounds of almost all categories can form nanosheets. The crystal structures of nanosheets also have a broad diversity, which reflects their composition. It is possible to obtain nanosheets with electrical characters ranging from dielectric to semiconducting and conducting properties. Such variation of the electric properties leads to differences in optical, photochemical, and magnetic properties. Similarly, nanosheet surface properties show behavior that ranges from hydrophilic (polar) to hydrophobic (nonpolar). Surface functionalities also show a wide variation, and many nanosheets can undergo surface chemical modifications to control their properties.
Because of the above-mentioned characteristics, inorganic nanosheets are now recognized as an important class of materials that offer novel advanced functionalities. Such materials have been developed, not only by fabrication of nanosheets, but also through fusion of nanosheets with other inorganic and organic substances. Thus, in this book we describe the preparation, structure, and functions of inorganic nanosheets themselves and various hybrid systems constructed by assembling them, as the materials chemistry of nanosheets. Applications of these materials have expanded in a range of the fields including various electronic, optic, and photochemical functions, adsorption and materials transportation, catalysis, and bio-related functions for medicine and pharmacy. The broadness of this range of applications reflects the diversity of compositions and properties of nanosheets.
1.2 Inorganic Nanosheets and Chemistry of Nanomaterials
Along with developments in nanotechnology, various inorganic particle systems possessing nm-level dimensions have drawn increasing interest. These materials have been investigated as functional fine particles that exhibit specific properties due to their nm-level size, and as building blocks for constructing higher order structures. Inorganic particles can be classified into 0D (nanodots, nanospheres), 1D (nanorods, nanowires, nanotubes), and 2D (nanosheets, nanoplates) particles based on their shape. Among these structures, considerable attention was initially been paid to isotropic 0D nanoparticles, which has expanded to anisotropic particles. In terms of anisotropic nanoparticles, many investigations into 1D matter have been performed and 2D nanoparticles have become an active area of study in the last decade. This situation is typically found in the trend shift of carbon allotrope research from fullerene to carbon nanotubes and most recently to graphenes.
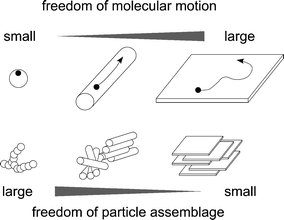
Using anisotropic nanoparticles as building blocks, we can develop novel functionally graded materials [). The surfaces of 2D particles are larger than those of 0D and 1D particles, and 2D surfaces can feature degrees of freedom within the plane, i.e., in both the x and y directions. Such structures allow for freer movement of electrons and molecules compared with 1D matter. Conversely, the freedom of assemblage of 2D particles is more restricted than those of the 0D and 1D particles, as a trade-off between the large freedoms of their surface morphology. Although 0D and 1D nanoparticles can organize into many types of structures, 2D nanoparticles basically assemble into layered structures. The layered structures provide 2D microspaces, restricted in the z (interlayer) direction but open in the x and y directions. Nevertheless, the interlayer spacing is often expandable to gain an additional degree of freedom in the z direction.
Fig. 1.3
Schematic representation of the characteristics of 0D ( dot ), 1D ( rod ), 2D ( plate ) particles. Circles and arrows indicate electrons or molecules and their transportation