Chapter 1
Chemoresponsive Materials: Introduction, Overview, Promises
HANS-JRG SCHNEIDER
FR Organische Chemie der Universitt des Saarlandes, D 66041 Saarbrcken, Germany,
1.1 Introduction and History
Chemoresponsive materials ideally combine sensors and actuators in the same unit. Living systems are regulated and thus maintained by responses to chemical signals, without the need for separate sensor and actuator devices. Living organisms are intrinsically both responsive and adaptive to external stimuli. A good example is the regulation of the insulin level for diabetics: man-made devices have relied until now mostly on measurement of glucose in blood and subsequent administration of insulin by injections. A smart solution, which is discussed in several chapters of the present book, is the use of a material which directly delivers insulin as a function of the continuously monitored glucose level in the blood. Like in nature one can hope to realize sensors and actuators within the same material, thus allowing self-regulating systems, or, in probably the most important application, selective drug delivery and targeting. How manifold applications of smart materials are for drug release is visible in many places of the book, such as in Chapter 2 by Schalley et al., 4 by Adams et al., 6 by Paulusse et al., 7 by Miyata, 8 by Payne et al., 9 by Harada et al., 10 by Lwik et al., 11 by Wickramasinghe et al., 13 by Vallet-Reg and Colilla, particularly for antitumor drugs, and 14 by Carrasco et al.
Many chemoresponsive materials are discussed in separate chapters of the present book, but not all could find their place. A number of these are therefore mentioned in this chapter; this applies in particular to systems that until now have mostly been described in solution, and played an essential role in the development of related smart materials. Also several new materials have only recently been shown on a larger scale to hold much promise for performance as both sensors and actuators.
Applications of chemoresponsive materials also encompass rapidly developing fields such as molecular electronics; Many advanced techniques of industrial and environmental importance rely on responsive smart materials, such as separation with membranes (see Chapter 11 by Wickramasinge), or those for surface modification (see Chapter 6 by Paulusse et al.).
The common feature of smart or intelligent materials is their ability to change their properties upon stimulation by external signals. Chemomechanical materials change volume and/or size by chemical stimulation (Chapter 2). Interestingly there exists also the reverse, namely materials that convert mechanical forces into chemical reactions
Smart materials responding to the chemical environment appeared rather late in the literature; the earliest demonstration of a macroscopic material change by external stimuli goes back to 1950, when scientists from different fields reported pH-induced stressable mechanical changes with a crosslinked polyacrylic acid polymer (
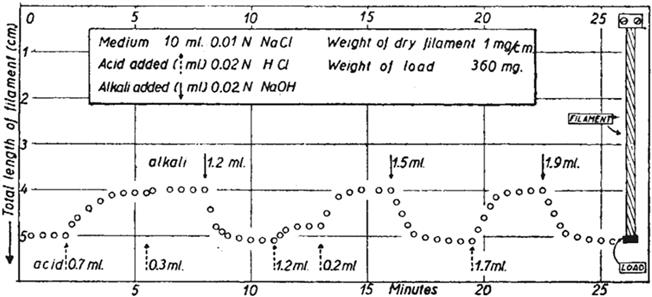
Figure 1.1 The historical first example of a chemomechanical material; reversible length change of a crosslinked polyacrylic acid filament by pH variation. Reproduced from ref. with permission from Springer Nature, Copyright 1950.
1.2 The Development of Molecular Switches and Machines
Ground breaking systems for chemically induced movements (see Chapter 15 by Shioi et al.) in the form of switches or even machines were first developed for complexes in solution. As they are not commonly regarded as materials they will be briefly discussed only in this chapter. Their development presented a most significant progress in chemistry in the last decades, which in 2017 was recognized by three Nobel prizes.
Fraser Stoddart was the first to introduce the ingenious use of rotaxanes In acid the complexation of the +NH2 ion with the crown ether dominates, under neutral conditions it is that with the permanently charged +NR ion.
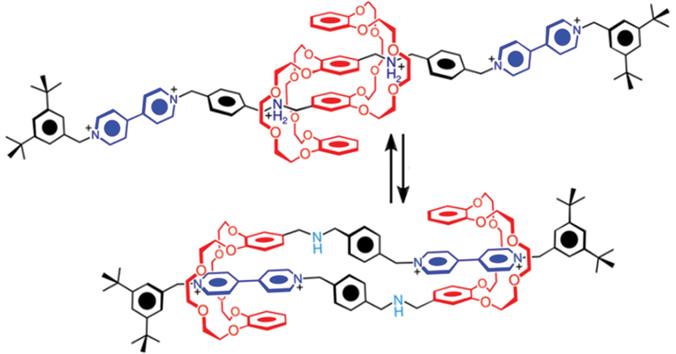
Figure 1.2 Actuation of movement of crown ether shuttles in a rotaxane by pH change. Adapted from ref. with permission from American Chemical Society, Copyright 2014.
With ligands that dimerize around a Fe2+ complex as linker one can obtain linear polymers with an average of 3000 repeating units, with a total length of several nanometres. The long poly[c2]daisy chains can amplify nanomotions of rotaxanes over several orders of magnitude, as in the single elements the contour lengths with the protonated part at B (15.9 m) and at the B unprotonated part (9.4 m) differ by 6.5 m (
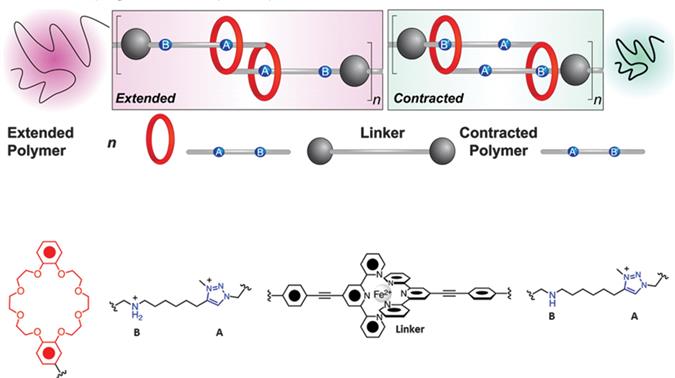
Figure 1.3 Contractile/extensile motions in rotaxane-based molecular muscles. In the polydaisy chains the crown ether (red) binds a low pH at station B and at higher pH at A. Adapted from ref. with permission from American Chemical Society, Copyright 2014.
Jean-Piere Sauvage opened up another fascinating way to design molecular machines by transition metal coordination.). With redox-active metals such as copper one can also trigger motions by oxidation/reduction cycles (see also Chapter 9 by Harada et al.).
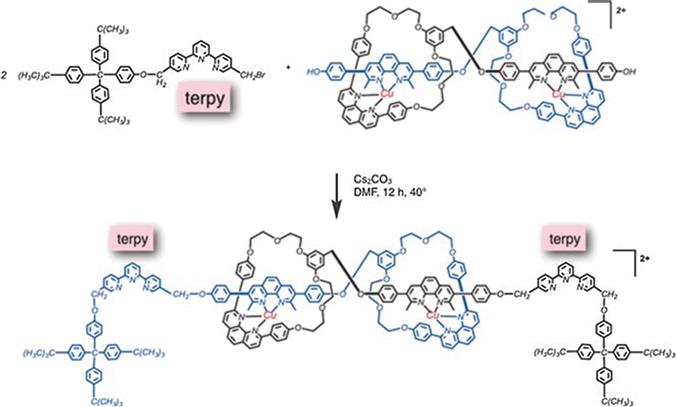
Figure 1.4 Two forms of a muscle-like [2]rotaxane dimer, with interconversion between both forms induced by metal exchange. Reproduced from ref. with permission from John Wiley & Sons, Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Ben Feringa introduced the use of cistrans isomerization in chiral overcrowded alkenes for unidirectional molecular motors induces by light, or e.g. by palladium ( Photochemical and thermal E/Z isomerization in optically active asymmetric biaryls were shown to lead to four different states and could be used to drive by light a unidirectional rotation around the central double bond.
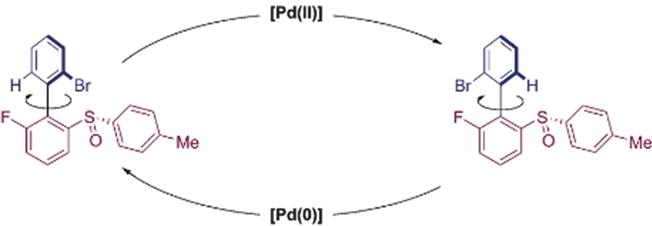
Figure 1.5 Chemical driven rotary motion: Pd-mediated rotation in a biaryl. Reproduced from ref. with permission from John Wiley & Sons, Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Supramolecular machines of the rotaxane type can be trapped within silica nanoparticles (SNPs, see also Chapter 13 by Vallet-Regi et al.), or mounted on a silica film and operate there as if they were in solution. shows a typical combination of (a) linear stalks anchoring rotaxanes to the surfaces of the SNPs, (b) gating rings in the form of macrocycles which encircle the stalks and trap the cargo, (c) an alternative ring binding site or weak, cleavable point along all the stalks that are susceptible to some specific stimulus such as an acid and force the rings to distance themselves from the pores, which then release the cargo, and (d) stoppers at the ends of the stalks.
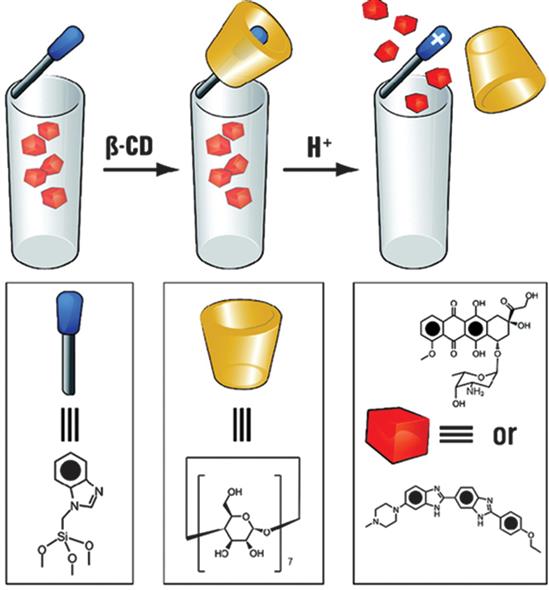
Next page