Springer International Publishing Switzerland 2016
David R. Crowe , Michael Morgan , Stephen Somach and Kara Trapp (eds.) Deadly Dermatologic Diseases 10.1007/978-3-319-31566-9_1
1. Angiosarcoma
Stephen Shuah , Michael B. Morgan 1, 2, 3 and Dylan Greeney
(1)
Director of Dermatopathology, Dermatology Specialists of Florida, Panama City, FL, USA
(2)
Staff Dermatopathologist, Advanced Dermatology and Cosmetic Surgery, Clermont, FL, USA
(3)
Professor of Dermatology, Michigan State College of Medicine, East Lancing, USA
Synonyms:
Hemangiosarcoma, lymphangiosarcoma, malignant hemangioendothelioma
Etiology:
Ultraviolet light, radiotherapy, lymphedema (Treves-Stewart syndrome), preexisting vascular malformations (Maffuccis syndrome)
Associations:
Maffuccis syndrome, BRCA 1 and BRCA 2 mutations , Klippel - Trenaunay , and neurofibromatosis type 1
Clinical:
Rapidly expanding bruise-like patch with indistinct borders , erythematous papules, violaceous nodules, may present with hemorrhage and ulceration if advanced
Histology:
Ill-defined anastomosing dermal network of atypical endothelial-lined spaces (most common) or defined diffusely arranged aggregates of epithelioid or spindled cells
IHC repertoire:
CD-31 (most sensitive and specific), CD-34, Ulex europaeus , factor VIII
Staging:
None for cutaneous disease
Prognosis:
Overall 5-year survival rate 10 %, resectable lesions localized to the skin have a 53.6 % - year survival rate
Adverse variables:
Size 5 cm; depth of invasion 3.0 mm; mitotic rate 3 HPF; positive surgical margins, recurrence, and metastases; age >; tumor located on the scalp and neck ; nonsolid growth pattern on histology ; epithelioid histology
Treatment:
WLE/XRT for localized disease, XRT for systemic disease, limited role for CTX
Angiosarcoma (AS), otherwise known as hemangiosarcoma, lymphangiosarcoma, or malignant hemangioendothelioma, is a malignant tumor derived from the endothelium that occurs in a variety of anatomic sites including the skin [].
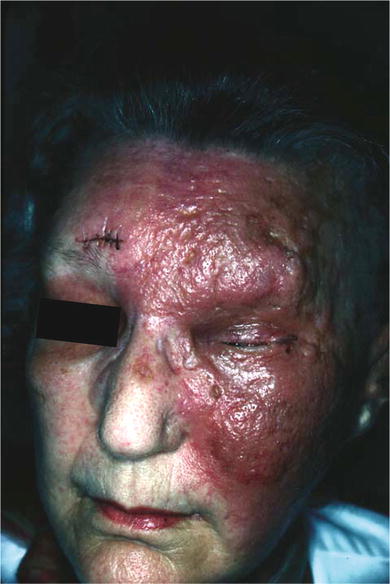
The clinical presentation is varied and dependent upon the various risk factor(s). Some lesions may appear benign or resemble infectious or inflammatory pathology. This variable presentation can delay proper initial diagnosis and treatment. Delay in diagnosis contributes to the overall poor prognosis of AS. AS masquerading as rosacea , rhinophyma , isolated eyelid edema , scarring alopecia , and chronic episodic facial edema can lead to misdiagnosis []. Although the scalp and face are most commonly afflicted, the ears , the neck , and the upper trunk may be involved as well. Lesions attributed to antecedent radiotherapy consist of rapidly growing papules and nodules classically located on the chest wall of women with a history of irradiated breast carcinoma. Radiotherapy-associated tumors may, however, arise in either sex and within the radiation field of a variety of anatomic sites. Most tumors arise following a 10-year or greater latent period. AS arising within a lymphedematous extremity is generally heralded by the development of a rapidly enlarging papule/nodule superimposed upon the brawny induration, which is typical of long-standing lymphedema. Most lesions develop an average of 10 years following surgery. Lesions associated with congenital lymphedema generally occur in younger patients who have experienced lymphedema for greater than 20 years. AS associated with preexisting vascular lesion(s) is characterized by rapid eccentric growth and epidermal ulceration.
Figure 1.1.
Violaceous plaque of angiosarcoma.
The histologic attributes of this lesion are varied. The most common pathologic alteration consists of a subtle increase in vascularity detected in the superficial and mid-dermis []. Although early lesions are confined to the dermis, well-developed lesions may extend laterally over a large expanse of dermis as well as invade deep into the subcutaneous fat and soft tissues. Microscopic extension of tumor is commonly seen well beyond what is deemed to be the clinical boundary of tumor.
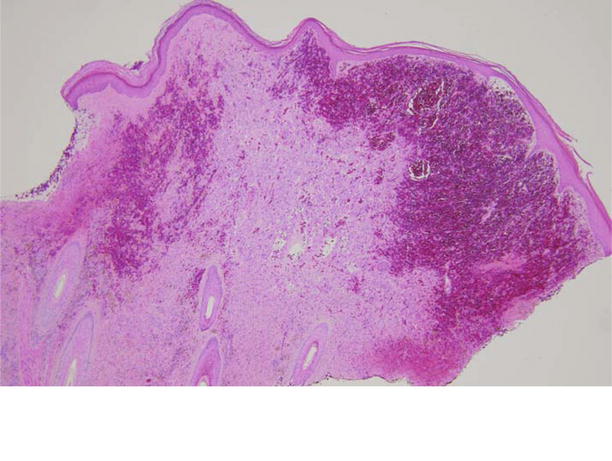
Figure 1.2.
Low-power photomicrograph depicting diffuse dermal hemorrhage.
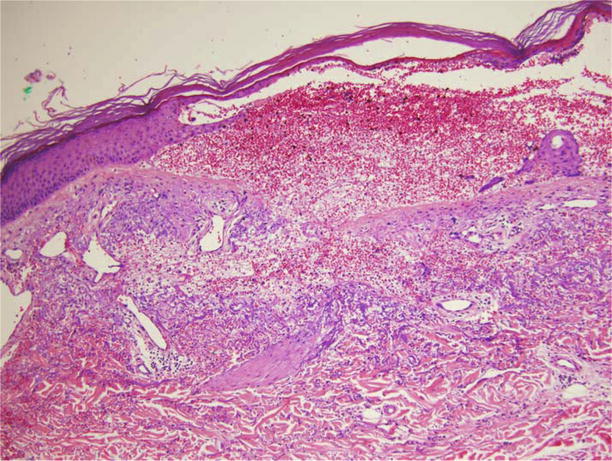
Figure 1.3.
Medium-power photomicrograph depicting subtle proliferation of endothelial-lined dermal vascular channels.
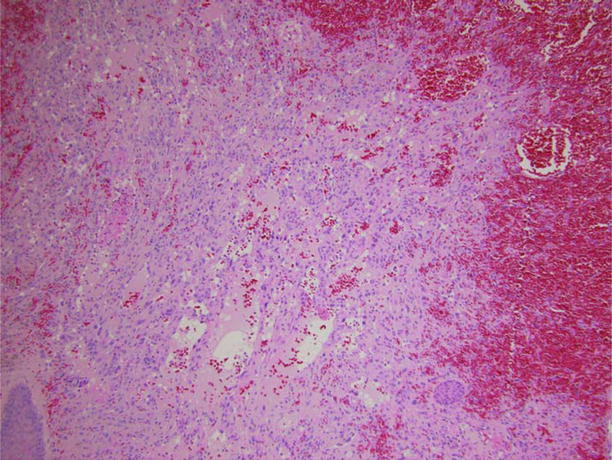
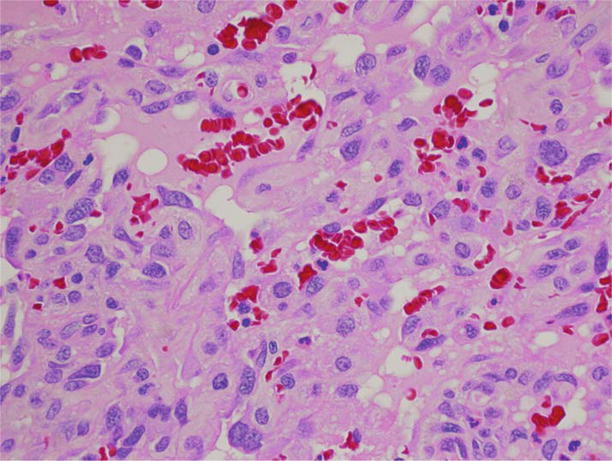
Figure 1.4.
Medium-power photomicrograph depicting deeper dermis with gaping vascular channels lined by atypical hyperchromatic endothelial cells.
Figure 1.5.
High-power photomicrograph depicting cytologic detail of vascular channels lined by atypical endothelial cells.
Special techniques that may be employed in confirmation of the diagnosis include electron microscopy and, increasingly, immunohistochemistry [].
Important entities to consider in the histologic differential diagnosis include benign entities such as the tufted angioma (TA) and targetoid hemosiderotic hemangioma (THH), low-grade vascular tumors of intermediate prognosis such as epithelioid hemangioendothelioma (EHA) and Kaposis sarcoma (KS), as well as malignant entities such as poorly differentiated carcinoma. THH consists of a superficial papillary dermal central focus of hobnailed vascular spaces and surrounding progressively inconspicuous and attenuated vascular channels. TA consists of discrete nests or tufts of epithelioid endothelia situated throughout the dermis. Endothelial atypia and/or extensive dermal or subcutaneous fat extension are not seen in these lesions. EHA is an uncommon tumor comprised of dermal and subcutaneous nests, strands, and diffusely arranged epithelioid cells often possessing intracytoplasmic lumina that contain erythrocytes. KS consists of a diffusely spindled cell population that characteristically forms slit-like vascular spaces and is punctuated by plasma cells and extracellular hyaline globules. Metastatic and poorly differentiated carcinoma may closely simulate AS. Epithelial connection, intercellular bridges, and glandular formation favor carcinoma. Difficult cases may require immunohistochemical characterization. Carcinomas should not stain with antibodies to CD-31.
AS is an aggressive tumor. It tends to recur locally, later metastasizing despite aggressive multimodal therapy. Wide surgical excision if possible is the most successful treatment option []. Generally, smaller tumors are more accessible to treatment with surgery. Other potential factors responsible for this observation include shorter clinical duration and limited vascular access with the attendant risk of metastases. Other favorable attributes recently shown to influence survival include average tumor mitotic rate of less than 3 per microscopic high-power field, a tumor depth of less than 3 mm, and absence of recurrence and metastases.