Springer International Publishing AG 2017
Jonathan A. Cotliar (ed.) Atlas of Graft-versus-Host Disease 10.1007/978-3-319-46952-2_1
1. Overview of Hematopoietic Cell Transplantation
Allogeneic hematopoietic stem cell transplantation (HSCT) is a form of immune therapy used to treat a variety of malignant and nonmalignant diseases. The procedure involves transfusion of multipotent hematopoietic stem cells derived from bone marrow, peripheral blood, or umbilical cord blood from a donor, usually matched in human leukocyte antigens (HLA). Immediately prior to HSCT, patients receive conditioning chemoradiotherapy to eliminate underlying hematologic malignant cells, and to sufficiently suppress the hosts immune functions for successful engraftment of donor hematopoietic cells. Following the conditioning regimen and HSCT, donor-derived hematopoietic recovery and immune reconstitution occur, during which patients require intensive supportive care, including prevention and treatment of complications such as infections and acute or chronic graft-versus-host disease (GVHD).
Currently, between 55,000 and 60,000 HSCTs are performed worldwide each year, including approximately 8000 in the United States alone [). Although allogeneic HSCT is the most effective and intensive therapy for hematologic disorders, there are significant barriers towards improving outcomes of HSCT, including transplant-related morbidity and mortality associated with acute and chronic GVHD, infection or delayed immune reconstitution, and regimen-related organ toxicities. In addition, despite intensive conditioning regimens and potent graft-versus-leukemia (GVL) effects, post-transplant relapse remains a significant cause of treatment failure. Thus, ongoing efforts are focused on improving patient selection criteria, preventing and treating GVHD and infection, and devising methods to reduce post-HSCT relapse of the underlying disease.
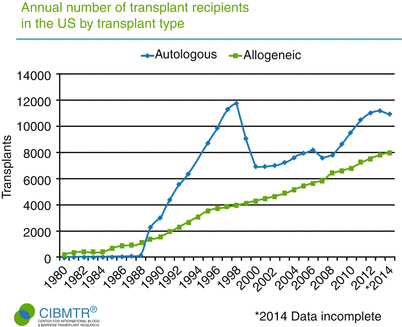
Fig. 1.1
Increasing numbers of patients are undergoing allogeneic hematopoietic stem cell transplantation (HSCT) every year ( Adapted from Pasquini and Zhu []; with permission )
Historical Perspective
Pioneering experimentation by Jacobson in the 1940s showed that mice were protected from the deleterious effects of radiation if their spleens were shielded with lead foils [].
Indications for Allogeneic Hematopoietic Cell Transplantation
Allogeneic HSCT is often the only potentially curative treatment for hematologic malignancies in an advanced stage or for relapsed disease. For early-stage disease such as acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first complete remission (CR1), the risk of relapse can be significantly reduced by allogeneic HSCT, but the medical decision is complex and has to be carefully balanced against the risk of transplant-related mortality. In general, allogeneic HSCT is considered for acute leukemia in CR1 with intermediate-risk or high-risk features, or cases that are beyond CR1. In the United States, AML/ALL and myelodysplastic syndromes (MDS) account for 65 % of patients who undergo allogeneic HSCT based on the most recent data provided by the Center for International Blood and Marrow Transplant Research (CIBMTR) []. With the introduction of targeted therapy with tyrosine kinase inhibitors for chronic myeloid leukemia (CML), HSCT is reserved only for patients with refractory disease. Refractory non-Hodgkins lymphoma, chronic lymphocytic leukemia (CLL), and nonmalignant diseases such as aplastic anemia, and paroxysmal nocturnal hemoglobinuria (PNH) comprise the remainder of indications for allogeneic HSCT.
The American Society of Blood and Marrow Transplantation (ASBMT) established a multiple-stakeholder taskforce to study role of HSCT in established disease states and identify emerging indications where HSCT may potentially be beneficial. This task force came out with a white paper in 2015 with clearly defined indications across disease states where HSCT has been shown to be of clinical benefit based on available clinical trial data. Published systematic evidence reviews or guidelines were used as the basis for recommendations to categorize indications for HSCT in pediatric and adult populations [].
Table 1.1
Diseases for which allogeneic and autologous hematopoietic stem cell transplantation is used []
Malignant |
Leukemia/preleukemia |
Chronic myeloid leukemia |
Myeloproliferative syndromes (other than chronic myeloid leukemia) |
Acute myeloid leukemia |
Acute lymphoblastic leukemia |
Juvenile chronic myeloid leukemia |
Myelodysplastic syndromes |
Therapy-related myelodysplasia/leukemia |
Kostmanns agranulocytosis |
Chronic lymphocytic leukemia |
Non-Hodgkins and Hodgkins lymphoma |
Multiple myeloma |
Solid tumors |
Breast cancer |
Neuroblastoma |
Sarcomas |
Ovarian cancer |
Small cell lung cancer |
Testicular cancer |
Nonmalignant |
Severe aplastic anemia |
Paroxysmal nocturnal hemoglobinuria |
Hemoglobinopathies |
Thalassemia major |
Sickle cell disease |
Congenital disorders of hematopoiesis |
Fanconi anemia |
Diamond-Blackfan syndrome |
Familial erythrophagocytic histiocytes |
Dyskeratosis congenita |
Schwachman-Diamond syndrome |
Severe combined immune deficiency and related disorders |
Wiskott-Aldrich syndrome |
Inborn errors of metabolism |
Acquired autoimmune diseases |
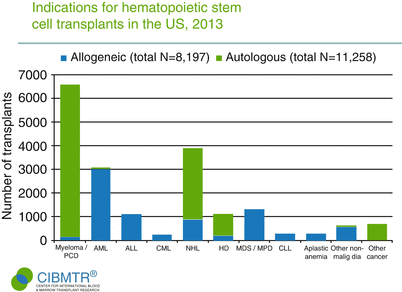
Fig. 1.2
Common indications for allogeneic and autologous HSCT (Adapted from Pasquini and Zhu []; with permission )
Sources of Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) can be found in a variety of human tissues, but for clinical purposes the most commonly used sources are peripheral blood, bone marrow, and umbilical cord blood. Each source has its unique advantages and drawbacks in clinical settings.
Peripheral Blood Mobilized Stem Cells