FROM ARSENIC TO ZIRCONIUM
Poems and Surprising Facts about the Elements
PETER DAVERN
FROM ARSENIC TO ZIRCONIUM
Copyright 2020 by Peter Davern.
All rights reserved. No part of this work may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage or retrieval system, without the prior written permission of the copyright owner and the publisher.
ISBN-10: 1-7185-0027-0
ISBN-13: 978-1-7185-0027-3
Publisher: William Pollock
Executive Editor: Barbara Yien
Production Editor: Laurel Chun
Cover Design: Octopod Studios
Interior Design: Octopod Studios
Illustrations: Peter Davern and Jenny Davern
Developmental Editors: Liz Chadwick and Edward Dodd
Technical Reviewer: John Elmsley
Copyeditor: Anne Marie Walker
Compositor: Kim Scott, Bumpy Design
Proofreader: Lisa Devoto Farrell
For information on distribution, translations, or bulk sales, please contact
No Starch Press, Inc. directly:
No Starch Press, Inc.
245 8th Street, San Francisco, CA 94103
phone: 1.415.863.9900;
www.nostarch.com
A catalog record of this book is available from the Library of Congress.
No Starch Press and the No Starch Press logo are registered trademarks of No Starch Press, Inc. Other product and company names mentioned herein may be the trademarks of their respective owners. Rather than use a trademark symbol with every occurrence of a trademarked name, we are using the names only in an editorial fashion and to the benefit of the trademark owner, with no intention of infringement of the trademark.
The information in this book is distributed on an As Is basis, without warranty. While every precaution has been taken in the preparation of this work, neither the author nor No Starch Press, Inc. shall have any liability to any person or entity with respect to any loss or damage caused or alleged to be caused directly or indirectly by the information contained in it.
To
Enda, Sam, and Jenny
ABOUT THE AUTHOR
Dr. Peter Davern is a lecturer in the Department of Chemical Sciences at the University of Limerick, Ireland.
ABOUT THE TECHNICAL REVIEWER
John Emsley is the author of several popular science books, including The Consumers Good Chemical Guide, which won the UK Royal Societys 1995 Science Book Prize. He was an academic chemist at Kings College London for 35 years before becoming a full-time science writer. He has been a Writer in Residence at Imperial College as well as Cambridge University.
PREFACE
Ive long been fascinated by the periodic table of the elements but have always had difficulty recalling some of the properties of even the more well-known elements. So to help me remember a few of the elements more prominent properties (and occasionally some of their more quirky ones!), Ive composed 93 short poems: one for each of the 92 elements from hydrogen to uranium on the periodic table, plus one additional poem to cover the 26 elements that follow uranium (known as the transuranium elements).
The poems were composed with reference to the various books, websites, journal articles, and reports listed in the Resources on .
The running order of the poems follows the normal sequence of the elements as they appear on the periodic table, which is determined by their atomic number: hydrogen is first with atomic number 1, then helium with atomic number 2, and so on to uranium with atomic number 92. The final poem covers the transuranium elements with atomic numbers 93 to 118. You can readily track down any element that interests you by consulting the index at the back of the book.
Each poem is accompanied by notes that explain each line. Oh, and one last detail: be sure to follow the pronunciation guide printed above certain words, chemical formulae, and so on; itll help you keep the poems meter!
I hope you find the poems enjoyable and useful.
ACKNOWLEDGMENTS
Sincerest thanks to the following people: Teresa Curtin, Sarah Hudson, Tom ODwyer, Tim Smyth, and, in particular, Peter Childs for their helpful comments and suggestions on my original draft text; Jenny Davern for baking the atomic structure cookies used as the basis for the cover illustration, and also for creating the illustrations for hydrogen, helium, boron, carbon, and nitrogen; Liz Chadwick and Edward Dodd for their rigorous and diligent editing of the book; Laurel Chun at No Starch Press for her assured management of the books production; Sam Davern for coming up with the initial concept for the cover illustration based on the atomic structure cookies imagery, and Derek Yee at No Starch Press for artfully transforming Sams concept into the final cover illustration; and John Emsley, esteemed author of Natures Building Blocks: An AZ Guide to the Elements, for kindly acting as the books technical reviewer.
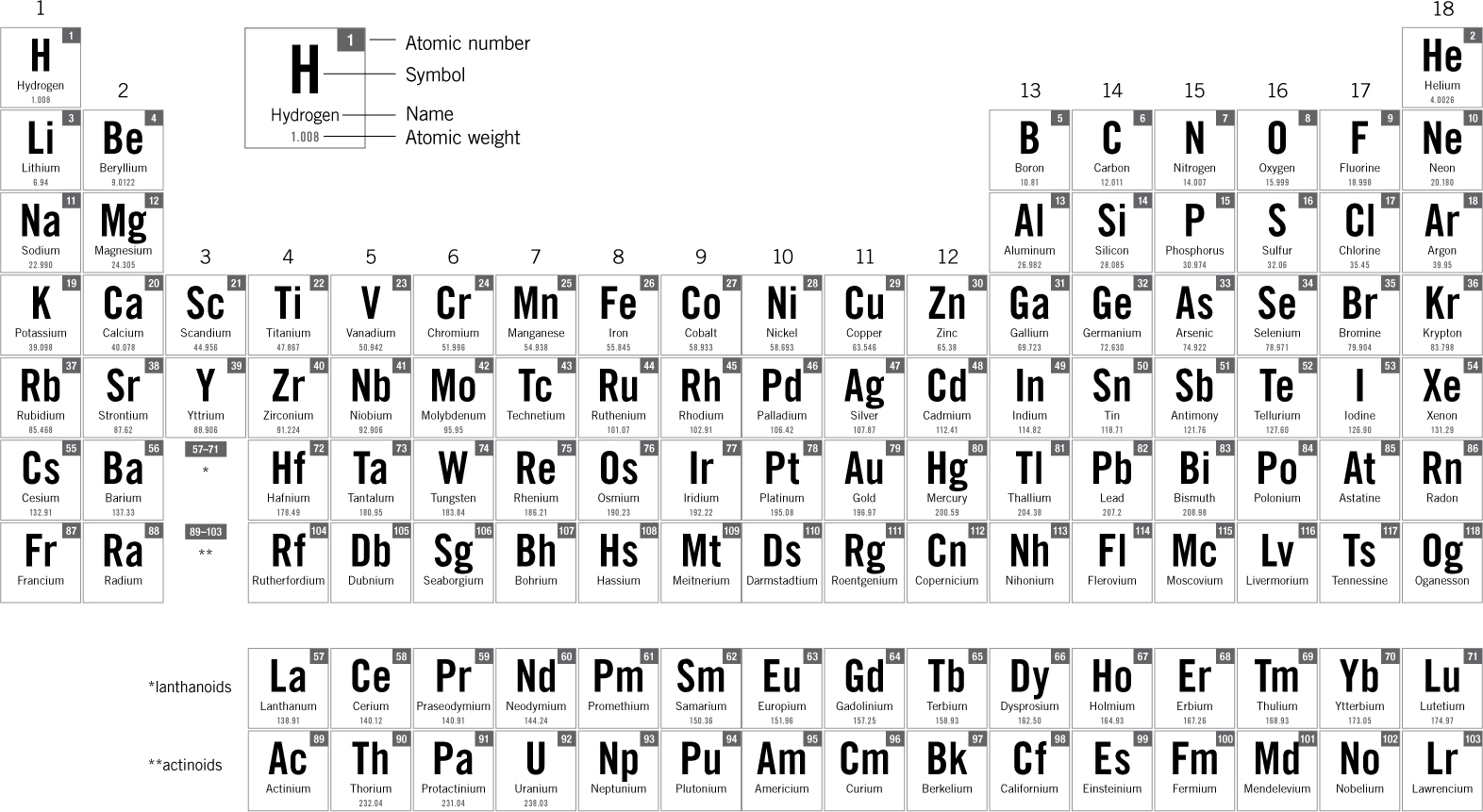
THE PERIODIC TABLE OF THE ELEMENTS
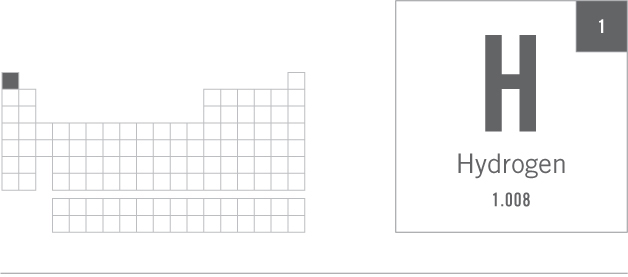
HYDROGEN, H
ROCKET MAN
On The Table it sits at the top,
THE FIRST ELEMENT OF THE PERIODIC TABLE
An element is a substance that cannot be broken down into simpler substances by ordinary chemical processes and is made of atoms of only one type. Elements are the fundamental materials of which all matter is composed.
Hydrogen is the simplest of all the elements in that its atoms consist of just one proton and one electron. It sits proudly atop the periodic table as element number 1. It was also the first element created during the Big Bang at the beginning of time.
Hydrogen gas was known long before it was recognized as an element. As far back as the 1500s, the Swiss alchemist Paracelsus (14931541) observed that bubbles of a flammable gas were produced when he added very fine pieces of iron (known as filings) to sulfuric acid (H2SO4). The Irish scientist Robert Boyle (16271691) spotted the same thing in 1671 when he dissolved iron filings in dilute hydrochloric acid (HCl); he called the gas a factitious air. It wasnt until 1766 that hydrogen was recognized as an element, by the eccentric yet brilliant English chemist Henry Cavendish (17311810).
Test tube lit it explodes with a pop!
Hydrogen, as the diatomic molecule H2, is the lightest of all gases. The time-honored laboratory test for detecting its presence is to light it in a test tube, at which point it burns so rapidly that it explodes with a satisfying, telltale pop.
Most abundant by far,
Hydrogen is by far the most abundant element in the universe, making up approximately three quarters of the universes mass. Its also the element that fuels our sun: every second roughly 600 million metric tons of hydrogen are converted to helium, accompanied (crucially for us!) by the conversion of around 5 million metric tons of matter to life-sustaining energy.