1. Photocatalytic CO2 Reduction
Abstract
In the context of finding sustainable and environmentally neutral alternatives to fossil fuels, there is much current interest in the production of chemicals that can be used as fuels using solar light ( solar fuels ). In the present chapter, we describe the fundamentals and the current state of the art for the photocatalytic reduction of CO2, making emphasis on the importance of the co-substrate (either water, hydrogen, or other electron donors), the differences of the process with respect to the photocatalytic hydrogen generation from water, and the importance to control the selectivity towards a single product of the many possible ones. After this part describing some basic issues of the photocatalytic CO2 reduction, some of the currently more efficient photocatalysts are described, delineating similarities and differences among those materials. The final section summarizes the main points of the chapter and presents our view on future developments in the field.
1.1 Solar Fuels: Concept and Importance
Modern societies consume an enormous amount of energy to perform their activities including transportation, industrial processes, and heating, among other main uses. The current scenario is based on the massive consumption of fossil fuels, including oil and natural gas, not only for transportation but also for the production of electricity and heating. Considering the limited resources on fossil fuels, it is clear that at the high current consumption rate these resources will become depleted, making the present energy source scheme unsustainable.
Besides sustainability, a second driving force to develop alternative energy sources is climate change and, specifically, atmospheric pollution by greenhouse effect gases. The limited resources of fossil fuels together with global warming concerns as well as geostrategic considerations derived from the uneven geographical distribution of oil and natural gas reserves have motivated a strong interest in developing alternative energy resources that should be characterized by sustainability and lack polluting emissions.
In this regard, one almost inexhaustible natural energy resource is sunlight reaching the Earths crust [].
In this context, one possibility would be to accumulate the energy of the sunlight into chemical compounds that could later be used in transportation or when required on demand []. However, the use of hydrogen as fuel cannot be readily implemented due to the lack of technology concerning its production, storage, and use. Due to the extremely low boiling point (253 C), hydrogen has to be handled as compressed gas, and this creates problems concerning filling fuel tanks and storage of enough energy to achieve autonomy in transportation.
Scheme 1.1
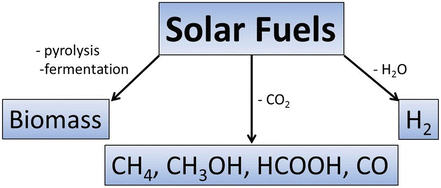
Most common chemicals considered as solar fuels
Besides hydrogen one alternative that is increasingly being considered is the use of C1 chemicals obtained by CO2 reduction, including methane, methanol, formic acid, and others. Although the use of solar fuels derived from CO2 has a major drawback in that CO2 having a greenhouse effect is formed in their combustion, the overall CO2 footprint should be neutral when these solar fuels are prepared from CO2 as feedstock [].
Besides hydrogen and CO2-derived chemicals, fuels from biomass are also considered in the broad sense as solar fuels since biomass is based on natural photosynthesis by green algae and plants [].
Solar fuels can be obtained in an indirect way by first converting solar energy into electricity and subsequently carrying out the electrochemical reduction of water or CO2 []. The most typical photocatalyst are solid semiconductors, such as some metal oxides, chalcogenides, and carbonaceous materials.
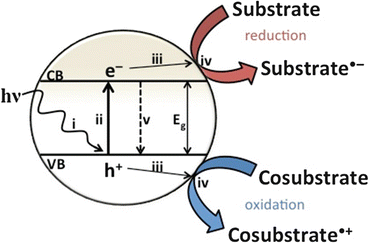
In photocatalysis photons, having energy higher than the bandgap of the semiconductor promotes excitation of electrons from the occupied state of highest energy to unoccupied states of lowest energy. This electronic excitation is generally described as charge separation since, very often in a semiconductor, electrons and/or holes are mobile and electronic excitation results in uncoupled electrons and holes (Scheme ).
Scheme 1.2
Elementary steps taking place in a photocatalytic cycle: light absorption (i), electron excitation (ii), electron and hole migration (iii), oxidation and reduction reactions (iv), or geminate electron recombination (v)
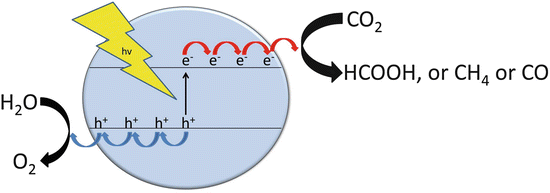
The state of charge separation can decay through different pathways, the most important ones being charge recombination of geminate electrons and holes generated in the same event of photon absorption or charge recombination after random migration of electrons or holes through the material [ illustrates the dual nature of charge-separated states as reducing and oxidizing agents. As briefly indicated above, for the sake of solar fuel production, the most wanted process is reduction of a substrate (H2O or CO2) by photogenerated electrons at the semiconductor to form the solar fuel. However, for the sake of fulfilling the electroneutrality principle, reduction in the photocatalytic process has to occur at the same reaction rate as the oxidation reaction by holes. This requirement of simultaneous oxidation and reduction of different chemicals in the same particle makes necessary that in the photocatalytic process, besides the targeted solar fuels, some oxidized compound has to be simultaneously formed.
Scheme 1.3
Schematic representation of an ideal photocatalyst performing CO2 reduction by water
In order to decouple oxidation and reduction at the semiconductor, achieving the maximum possible efficiency in the reduction semireaction leading to solar fuels, it is very common that model studies use a sacrificial agent whose role is to quench efficiently at a high rate photogenerated holes, allowing the study of the reduction process as the rate-limiting step. Preferred hole quenchers in this type of photocatalytic studies are tertiary amines, particularly triethanolamine and triethylamine, and alcohols, especially methanol and ethanol, and also inorganic ions such as sulfide, sulfite, and nitrate. To differentiate those photocatalytic experiments in the absence of a sacrificial electron donor with those having present these reagents, it is common to indicate the term overall to denote that photocatalytic oxidation and reduction of the same substrate are simultaneously carried out without any sacrificial electron donor (Scheme ). Photocatalytic generation of solar fuels has the advantage with respect to indirect strategies based on electrolysis in that they require much less infrastructure and lower capital investment in equipment, and therefore, it could be more amenable for on-site implementation in small- or medium-sized plants, rather than application in large facilities.