Astronauts aboard Apollo 17 took this famous photo of Earth, known as the Blue Marble, in 1972. It created a global awareness of the fragility of Earth and helped fuel the environmental movement.
D id you know that atoms are so small that if we took all the atoms in a cup of water and made them the size of a marble, Earth would be covered in more than 50 miles (80 km) of marbles? Atoms are the building blocks of all matter on Earth, and understanding atoms is central to modern life. Through chemistrythe study of atoms and chemical processesresearchers are able to do many things. They can design new medicines, make smaller and longer-lasting batteries, and improve fuel efficiencies of cars, planes, and other vehicles.
While chemical processes can make our lives easier and more fun, they can also pollute the environment around us. Our shared planet faces many environmental challenges. Understanding the chemistry that underlies these issues is a key part of coming up with solutions. The field of environmental chemistry focuses on understanding the most common pollutants; how they pollute; how they get into our food, water, and air; and what the consequences to human, plant, and animal health are. Just as critical is that environmental chemistry also focuses on solutions.
Down to Basics
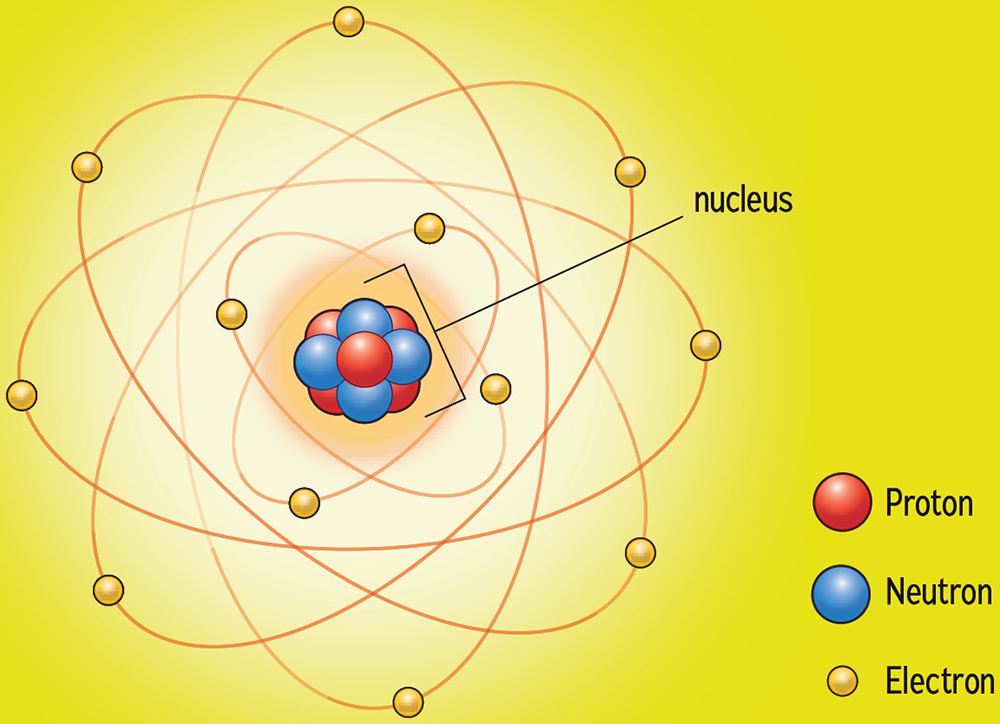
Atoms are tiny spheres composed of three types of subatomic particles. In the center of each sphere is an incredibly small nucleus. The nucleus is made of neutrons (with no electrical charge) and protons (with a positive charge). Whizzing around the nucleus are negatively charged electrons. All atoms are neutral and have no overall electrical charge. They have equal numbers of protons and electrons. But every element has a different number of subatomic particles. Hydrogen has the fewest, with just one proton, one electron, and zero neutrons. At the other extreme is oganesson, which has 118 protons, 118 electrons, and 176 neutrons. The number of protons and the number of electrons in an atom is the atomic number, often abbreviated as the letter Z . The atomic mass number, A, is the combined number of protons and the neutrons. For oganesson, Z = 118 and A = 294. For hydrogen, A and Z are both 1.
Basic Atom
The basic subatomic particles of an atom are protons, neutrons, and electrons. Each chemical element, from hydrogen to oganesson, has a different number of subatomic particles.

Russian chemist Dmitri Mendeleev organized his periodic table by the properties and mass of known elements at the time. He understood that the physical and chemical properties of elements were related to their atomic mass. So he put groups of similar elements in the same vertical columns. His table was so accurate that it predicted the existence and properties of elements that had yet to be discovered and allowed room to insert them once they were.
In 1869 Russian chemist Dmitri Mendeleev published a groundbreaking infographic. At the time, scientists knew of about fifty-six types of chemical elements. But no one had come up with a good way to arrange them into an easy, practical table. Mendeleev came up with a very elegant and useful visual way of organizing all the known elements. Even though it was not immediately accepted, his ideas formed the basis of the modern periodic table.
In the periodic table, each of the elements in a particular column, also called a group, or family, is abbreviated by a one- or two-letter symbol. The periodic table has eighteen groups. Each of the elements is numbered, referring to the number of protons in that atoms nucleus. This number is called the elements atomic number (Z). The elements in each group have similar properties. For example, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) are in group 1. They are all solids that react violently with water. On the far side of the periodic table is group 18 with helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). They are all unreactive gases. Oganesson is also in group 18, but it is synthetic (manufactured by humans) and only exists for a fraction of a second.
The periodic table is also divided into three main sectionsmetals, nonmetals, and metalloids. Metals make up most of Earths known atoms and are in the center of the table. Metals conduct heat and electricity and melt at very high temperatures. Some of the metals in the lower portions of the periodic table are very dense and are called the heavy metals. For example, mercury (Hg) is 13.5 times denser than water, and lead is 11.3 times denser than water. Mercury is the only liquid metal. It is so dense that a laptop would float on top of liquid mercury. Some of the heavy metals, such as mercury, lead, and arsenic, are very toxic. Other heavy metals, such as silver, gold, and ruthenium, are relatively harmless. Some metals such as iron, zinc, and cobalt are essential nutrients, and we would die without them in our diet.
Nonmetals are on the right side of the table. They do not conduct heat and electricity and tend to be brittle solids. Examples of nonmetals are sulfur, carbon, and silicon. Metalloids are elements that resemble both metals and nonmetals. They look like metals and are shiny. But they behave like nonmetals because they dont conduct electricity and are brittle.