You know all about Schrdingers cat, but how about his equation? How do lasers, transistors and electron microscopes work? What are the perils of renormalization? What makes a fluid a superfluid? And what will a quantum computer be capable of? Discover the history of some of sciences greatest discoveries and ponder the future of physics and technology with this fascinating guide to quantum theory.
30-Second Quantum Theory tackles a mindbendingly mysterious area of physics, introducing the 50 most significant quantum quandaries and ideas. At a time when the quantum physics of electronics is an everyday essential and new quantum developments make headline news, you will visit parallel worlds, ride wave theory and learn enough to talk with certainty about Uncertainty Principle and to untangle the mysteries of quantum entanglement.
30-SECOND
QUANTUM THEORY
The 50 most thought-provoking quantum concepts, each explained in half a minute
Editor
Brian Clegg
Contributors
Philip Ball
Brian Clegg
Leon Clifford
Frank Close
Sophie Hebden
Alexander Hellemans
Sharon Ann Holgate
Andrew May

First published in the UK in 2014 by
Icon Books Ltd
Omnibus Business Centre
3941 North Road, London N7 9DP
email: info@iconbooks.net
www.iconbooks.net
2014 by Ivy Press Limited
The editor and contributors have asserted their moral rights.
No part of this book may be reproduced in any form, or by any means, without prior permission in writing from the publisher.
This book was conceived, designed and produced by
Ivy Press
210 High Street, Lewes,
East Sussex BN7 2NS, U.K.
www.ivypress.co.uk
Creative Director Peter Bridgewater
Publisher Susan Kelly
Editorial Director Caroline Earle
Art Director Michael Whitehead
Project Editor Jamie Pumfrey
Designer Ginny Zeal
Illustrator Ivan Hissey
Glossaries Text Brian Clegg
Print ISBN: 978-1-848316-66-9
eBook ISBN: 978-1-848317-44-4
Colour origination by Ivy Press Reprographics
CONTENTS
INTRODUCTION
Brian Clegg
The physics we are taught at school can be, frankly, rather boring. It is worthy, 19th-century science necessary, certainly, but hardly earth-shattering. What a shame we dont introduce schoolchildren earlier to the more exciting bits: and there is nothing more thrilling and mind-boggling in all of science than quantum theory.
Small things
The idea that matter, stuff, is made up of tiny fragments goes all the way back to the ancient Greeks, but the ideas of the atomists (the name atom comes from the greek word atomos, uncuttable) were largely sidelined by the four element theory that considered everything to be made up of earth, air, fire and water. By the end of the 19th century, atoms had made a comeback as useful concepts in chemistry and physics, but no one was quite sure what they were or how they worked. Some even doubted they existed. To the surprise of the scientists, atoms not only proved to be a reality, but these tiny components of everything from a human being to a speck of dust also behaved more strangely than anyone could expect.
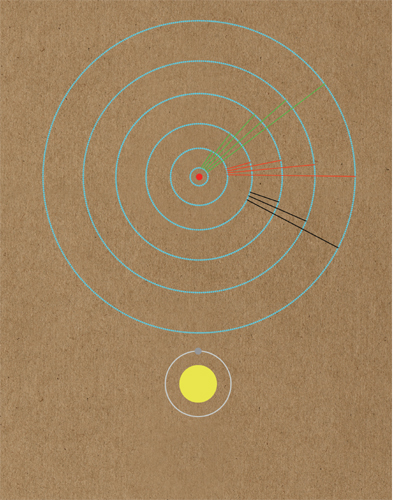
It was initially assumed that atoms and their component parts would behave just like much smaller versions of the ordinary things that we see around us. Therefore, scientists thought that atoms would fly through the air just as a tennis ball does, if on a smaller scale. When it was discovered that atoms had internal structure, it was first theorized that they might be like plum puddings, with negative charges scattered through a positive body, but the revelation that most of their mass was in a central nucleus made it seem obvious that an atom was like a miniature solar system.
The quantum revolution
Unsettlingly for the old guard of physics (though delightfully for the rest of us), this picture turned out to be impossible to maintain. An atom built like a solar system would not be stable, and quantum particles refused to behave as predictably as a tennis ball. As quantum theory was developed it became clear that there was a fundamental difference between the microscopic and the macroscopic world. Tennis balls followed clear paths depending on their mass and the forces acting on them. But quantum particles could only be given probabilities of behaving in a particular way. At the heart of their behaviour was randomness and before they were observed it was never possible to be certain exactly what they were up to.
This horrified Einstein, inspiring him to write I find the idea quite intolerable that an electron exposed to radiation should choose of its own free will, not only its moment to jump off, but also its direction. In that case, I would rather be a cobbler, or even an employee in a gaming house, than a physicist, and produced his famous comments along the lines of God does not play dice. But others were fascinated.
The great American quantum physicist Richard Feynman said Im going to describe to you how Nature is and if you dont like it, thats going to get in the way of your understanding it [Quantum theory] describes Nature as absurd from the point of view of common sense. And it agrees fully with experiment. So I hope you can accept Nature as she is absurd. I hope that this book will give you a chance to experience and echo Feynmans enjoyment of the sheer strangeness and absurdity of the quantum world.
Dividing the quantum
There surely can be few topics that are more suited to being divided up in bite-sized, easily digested chunks than quantum theory. The 50 articles that follow are split into seven sections, each taking on a part of this significant undertaking. We begin, aptly enough, with The Birth of the Theory , which describes how the classical view that atoms were no different from the objects we see around us was undermined by observation and how the endeavour to find a way that atoms could be stable at all required a very different approach.
From the origins we move on to The Essentials , the key components of quantum theory, some of which, like Heisenbergs Uncertainty Principle, have escaped the confines of physics to become part of popular culture. With these fundamentals in place we can open up the science of practically all of our everyday experience, The Physics of Light & Matter . Quantum electrodynamics, the theory that explains everything from the way sunlight can warm us to the reason why you dont fall straight through your chair, required a whole new way of looking at the quantum world and has become the most successful theory ever in terms of the accuracy with which it predicts what is observed.
From here we move onto some key Quantum Effects & Interpretation , explaining how we can both see through and see a reflection in a window, or how quantum tunnelling keeps the Sun working and the thorny matter of quantum interpretation. Quantum theory is almost unique in this respect. It is excellent at predicting what we observe, but no one is sure exactly what the theory itself represents. Descriptions like the Copenhagen, Many Worlds and Bohm interpretations attempt to put what is observed into a framework that explains why such observations are made, yet we have no way to distinguish between these options, choosing on personal preference rather than good scientific logic.
Next page














