Rainer Hofmann-Wellenhof , Giovanni Pellacani , Joseph Malvehy and Hans Peter Soyer (eds.) Reflectance Confocal Microscopy for Skin Diseases 2012 10.1007/978-3-642-21997-9_1 Springer-Verlag Berlin Heidelberg 2012
1. The Confocal Story
Abstract
Dermatologic diagnosis is primarily based on interpretation of morphological information by visual inspection, confirmed by histopathological diagnosis if necessary. The challenge is to establish a correct diagnosis and to identify all malignant lesions while minimizing unnecessary surgical procedures. In this context, several non-invasive imaging modalities have emerged in recent years that are aimed at increasing accuracy of in vivo diagnosis. Of those, reflectance confocal microscopy has shown the most promising results. In vivo reflectance confocal microscopy produces horizontal images of the skin at a cellular resolution from the surface to the upper dermis. It enables to visualize tissue in its physiological state avoiding retraction bias due to fixation, staining and sectioning procedures that are a prerequisite to conventional light histopathology. Moreover, RCM enables observation of changes over time.
Dermatologic diagnosis is primarily based on interpretation of morphological information by visual inspection, confirmed by histopathological diagnosis if necessary. The challenge is to establish a correct diagnosis and to identify all malignant lesions while minimizing unnecessary surgical procedures. In this context, several non-invasive imaging modalities have emerged in recent years that are aimed at increasing accuracy of in vivo diagnosis. Of those, reflectance confocal microscopy has shown the most promising results. In vivo reflectance confocal microscopy produces horizontal images of the skin at a cellular resolution from the surface to the upper dermis. It enables to visualize tissue in its physiological state avoiding retraction bias due to fixation, staining and sectioning procedures that are a prerequisite to conventional light histopathology. Moreover, RCM enables observation of changes over time.
The principle of confocal scanning microscopy relies on single-point illumination and a pinhole in an optically conjugate plane (hence the name confocal) in front of the detector to eliminate out-of-focus signal and on scans of a given specimen point by point (see ). The interest in the use of reflectance confocal microscopy for imaging of human skin and thus the number of related publications has risen sharply in recent years. A search of MEDLINE (search phrases: reflectance confocal microscopy & skin) in 2000 would have displayed ten publications on RCM. By November 2010 that number has grown to more than 150 publications displayed.
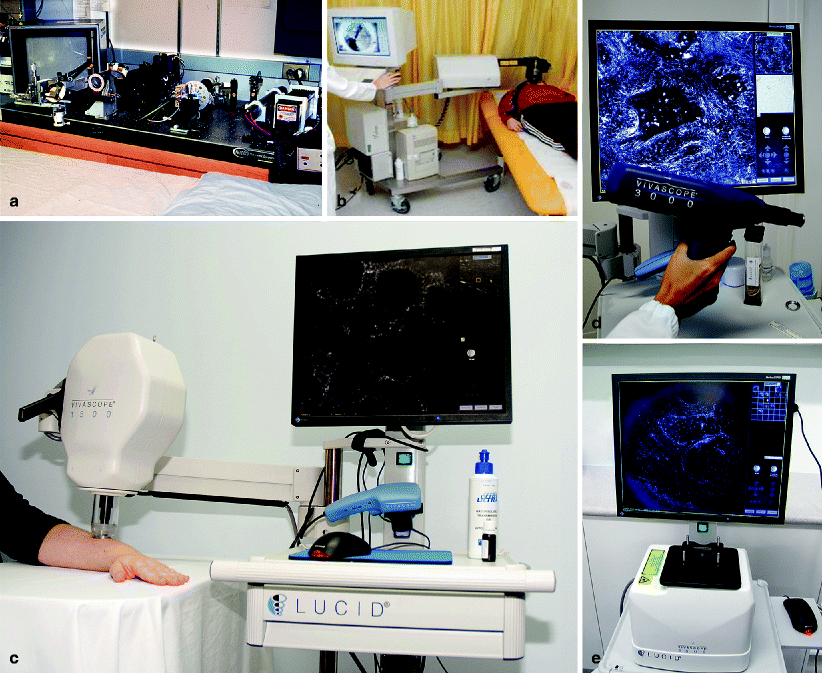
Fig. 1.1
Technical advancements have helped to decrease the size of laser scanning confocal microscopes from spacious machines down to a movable device allowing for clinical imaging at the bedside. ( a ) First confocal microscope for in vivo imaging, Wellman Labs, Dermatology Department, MGH, Harvard Medical School in 1995. ( b ) VivaScope 1000, Dermatology Department, Graz, Austria, in 2005. ( c ) VivaScope 1500, Dermatology Department, Brisbane, Australia, in 2008. ( d ) VivaScope 3000 handheld device, Dermatology Department, Modena, Italy, in 2010. (e) VivaScope 2000 for ex vivo imaging My-Lab, Brisbane, Australia, in 2010
Confocal laser scanning microscopy (CLSM) may be used either in reflectance mode (RCM) or fluorescence mode (FLSM). In the latter, imaging relies on endogenous and exogenous fluorophores. Fluorescent dyes are used as exogenous sources of contrast. A laser beam is used to excite the fluorophores. The company OptiScan Ltd. (Melbourne, Australia) developed a fluorescence confocal microscope for imaging of human skin. A small handheld scanner with an optical fiber is used to illuminate the tissue and to detect fluorescence signals. A single image of 250 250 m can be obtained at different depths (z-stack) but it does not provide imaging of a bigger horizontal field of view (xy-mosaic).
Preliminary projects in skin imaging have not led to a breakthrough in clinical use probably due to limited imaging depth and the necessity to inject exogenous flourophores [], and devices that combine both modes for use in humans have been recently developed.
Reflectance mode relies on inherent variations in refractive indices of skin structures [). Furthermore, this device is the first to have an integrated dermoscopic camera, underlining the importance of RCM as a natural link between dermoscopy and histopathology. By using the dermoscopic image as a gross map to navigate to a region of interest which can be viewed in real time with the RCM, a correlation between dermoscopy and confocal images is provided. Further developments are the VivaScope 3000, a handheld confocal microscope for imaging of difficult to access areas, and the ex vivo VivaScope 2500 designed for imaging of excised tissue, especially in Mohs surgery as well as Vivascope 1500 Multilaser, which combines FLSM and RCM modes.
Melanin and melanosomes are strong source of contrast that appear bright in the images []. Other fields of confocal research include non-melanocytic lesions and inflammatory skin diseases. Furthermore, RCM offers a unique tool to assess dynamic changes and to monitor lesions over time. As lesions often expand over what can be perceived with the naked eye, RCM can help to detect the true surgical margins preoperatively. All these applications will be addressed in this book.
As in every morphologic discipline the interpretation and thus description of structures is subjective and may depend on the socio-cultural background of the observer. In such a young discipline as RCM, this has led to a variation of terms used for similar features that may confound the novice. In order to overcome this, a consensus for standard RCM terminology has been published in 2007 []. Features of this website comprise a tutorial displaying and explaining the most common features of benign and malignant skin lesions and a training platform to review single features and to diagnose lesions in a reproduced clinical setting. Although the future cannot be predicted, we are confident that morphologic evaluation is irreplaceable in dermatologic diagnosis and RCM is predestined to enhance diagnosis and move it closer to the bedside. Based on the fast-paced developments in the field of biophotonics, we foresee a future with more powerful and user-friendly confocal devices. The confocal story has just begun.
References
Minsky M (1988) Memoir on inventing the confocal scanning microscope. Scanning 10:128138 CrossRef
Psaty EL, Halpern AC (2009) Current and emerging technologies in melanoma diagnosis: the state of the art. Clin Dermatol 27:3545 PubMed CrossRef
Gonzalez S (2009) Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr 100(Suppl 2):5969 PubMed CrossRef
Swindle LD, Thomas SG, Freeman M, Delaney PM (2003) View of normal human skin in vivo as observed using fluorescent fiber-optic confocal microscopic imaging. J Invest Dermatol 121:706712 PubMed CrossRef