Chao-Min Cheng - In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices
Here you can read online Chao-Min Cheng - In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2015, publisher: Springer, genre: Home and family. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices
- Author:
- Publisher:Springer
- Genre:
- Year:2015
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Addressing the origin, current status, and future development of point-of-care diagnostics, and serving to integrate knowledge and tools from Analytical Chemistry, Bioengineering, Biomaterials, and Nanotechnology, this book focusses on addressing the collective and combined needs of industry and academia (including medical schools) to effectively conduct interdisciplinary research.
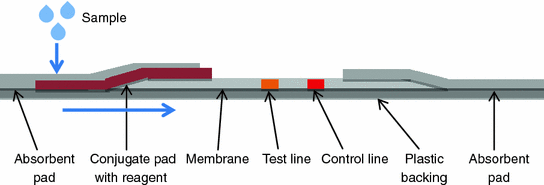
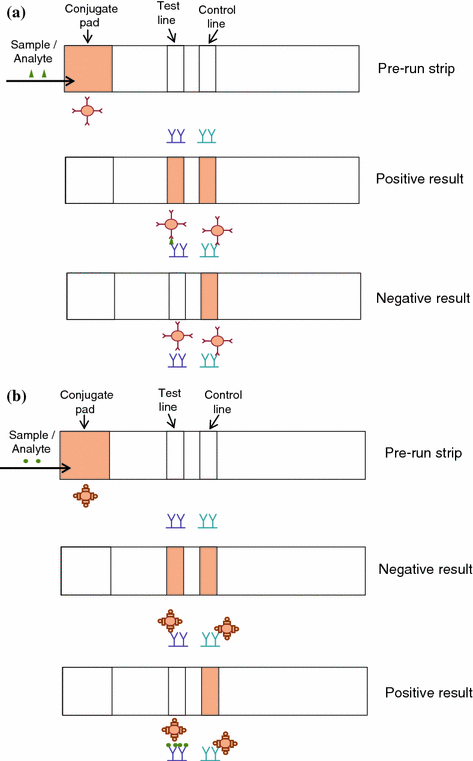
In addition to summarizing and detailing developed diagnostic devices, this book will attempt to point out the possible future trends of development for point-of-care diagnostics using both scientifically based research and practical engineering needs with the aim to help novices comprehensively understand the development of point-of-care diagnostics. This includes demonstrating several common but critical principles and mechanisms used in point-of-care diagnostics that address practical needs (e.g., disease or healthcare monitoring) using two well-developed examples so far: 1) blood glucose meters (via electrochemistry); and, 2) pregnancy tests (via lateral flow assay).
Readers of this book will come to fully comprehend how to develop point-of-care diagnostics devices, and will be inspired to contribute to a critical global cause the development of inexpensive, effective, and portable in vitro diagnostics tools (for any purpose) that can be used either at home or in resource limited areas.
Chao-Min Cheng: author's other books
Who wrote In-Vitro Diagnostic Devices: Introduction to Current Point-of-Care Diagnostic Devices? Find out the surname, the name of the author of the book and a list of all author's works by series.