Michael J. Murphy (ed.) Current Clinical Pathology Molecular Diagnostics in Dermatology and Dermatopathology 10.1007/978-1-60761-171-4_1 Springer Science+Business Media, LLC 2011
1. Introduction to Molecular Diagnostic Testing in Dermatology and Dermatopathology
Abstract
The complete sequencing of the human genome has ushered in an era of medical advances that was previously unimaginable. Scientists are continually discovering novel genetic and epigenetic mechanisms that are associated with human disease states and therapeutic responses. The ability to determine the underlying defect(s) in single-gene (Mendelian) diseases, many of which are rare, has improved both diagnosis in symptomatic patients and risk prediction of future disease in asymptomatic individuals. Potential applications of genomic discoveries include: (1) development of carrier, screening and diagnostic tests for single-gene disorders; (2) evaluation of several genetic loci in an effort to construct disease susceptibility profiles for non-Mendelian diseases, based on multiple gene and/or geneenvironment relationships; and (3) pharmacogenomic testing to predict druggenome interactions [1]. It has been estimated that 5% of the 25,000 genes in the human genome are of diagnostic significance; therefore, the potential exists to develop 1,500 gene-based tests [2]. With regard to dermatologic conditions, exciting research is emerging and new applications are now being incorporated into clinical practice. Molecular diagnostic tests are transforming laboratory medicine and patient care, and becoming indispensable for physicians involved in the management of skin diseases, including dermatologists and dermatopathologists. Nucleic acid-based testing is becoming a crucial diagnostic tool, not only in the setting of inherited disorders (i.e., genodermatoses), but also for a wide variety of cutaneous solid and hematopoietic tumors, inflammatory dermatoses, and infectious conditions. In view of the increasing numbers of molecular diagnostic articles published in the dermatology literature, and potential application of these methodologies in clinical practice, a basic knowledge of the principles of molecular diagnostics is now essential for the physician who specializes in the diagnosis and/or treatment of skin diseases. Figure 1.1 illustrates the integration of research and diagnostic strategies in the study of skin diseases.
O brave new world, he repeated. O brave new world that has such people in it. Lets start at once.
Aldous Huxley, Brave New World (1932)
The complete sequencing of the human genome has ushered in an era of medical advances that was previously unimaginable. Scientists are continually discovering novel genetic and epigenetic mechanisms that are associated with human disease states and therapeutic responses. The ability to determine the underlying defect(s) in single-gene (Mendelian) diseases, many of which are rare, has improved both diagnosis in symptomatic patients and risk prediction of future disease in asymptomatic individuals. Potential applications of genomic discoveries include: (1) development of carrier, screening and diagnostic tests for single-gene disorders; (2) evaluation of several genetic loci in an effort to construct disease susceptibility profiles for non-Mendelian diseases, based on multiple gene and/or geneenvironment relationships; and (3) pharmacogenomic testing to predict druggenome interactions [ illustrates the integration of research and diagnostic strategies in the study of skin diseases.
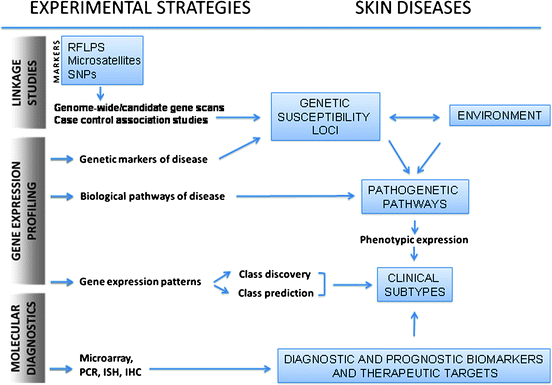
Fig. 1.1
Integration of research and diagnostic strategies in the study of skin diseases. Linkage studies facilitate an understanding of genetic markers and susceptibility loci. Microarray-based gene expression analysis enables a more thorough understanding of genegene and geneenvironment interactions that are involved in disease induction and pathogenesis. Identification of disease-specific genetic signatures should provide a better classification of disease at the molecular level, redefining both known and unknown subtypes of disease. Data is incorporated into molecular diagnostic strategies for risk prediction, identification of diagnostic and prognostic biomarkers, and discovery of therapeutic targets of disease. RFLPs restriction fragment length polymorphisms, SNPs single nucleotide polymorphisms, PCR polymerase chain reaction, ISH in situ hybridization, IHC immunohistochemistry (Courtesy of Dr. Zendee Elaba, department of Pathology, Hartford Hospital, Hartford, CT, USA: Adapted from Dudda Subramanya et al. [])
Essentially, molecular diagnostic testing involves the analysis of nucleic acid (DNA and/or RNA), using a wide variety of technologies. Simplistically, this field can be divided into disease diagnostics (i.e., risk prediction, disease identification, and recurrence detection) and companion diagnostics (i.e., drug responders, titration of efficacy, and patient selection for clinical trials) []. Most molecular assays consist essentially of three steps: extraction and purification of nucleic acid, amplification of the target sequence, and detection of the amplified product. The goal of POC testing is to streamline and miniaturize these processes, in order to develop hand-held devices for bed-side testing of those conditions (such as life-threatening infections) where rapid diagnosis is necessary for initiation of appropriate therapy.
The purpose of this book is to introduce the basics of molecular biology and molecular diagnostic methods most commonly used in the clinical laboratory, with an emphasis on the concepts and potential applications that are most relevant to dermatologic conditions. The contents of this book will be of particular interest to dermatologists and dermatopathologists, as well as anatomic pathologists and other physicians/scientists who have an interest in skin disorders. A broad range of cutaneous pathology is covered. In addition to chapters on infectious and inflammatory lesions, cutaneous lymphoproliferative disorders, melanocytic tumors, non-melanoma skin cancers and genodermatoses, the book also includes discussions of other dermatologic conditions, including cutaneous sarcomas and soft tissue proliferations, metastatic tumors, wound healing disorders, and alopecias. The application of pharmacogenetics and pharmacogenomics in patient management, and some of the regulatory, legal, coding, billing, reimbursement, and ethical considerations associated with molecular diagnostic testing in dermatology and dermatopathology are also outlined. The integration of newer diagnostic, staging, and prognostic molecular techniques with traditional clinical- and histopathological-based approaches is described, and a broad and comprehensive outline of current clinically relevant applications of these methodologies in dermatologic conditions is provided. Molecular studies that primarily investigate the pathogenesis of skin diseases (and can be found in other texts) have been largely excluded or have minimal discussion, unless they also have direct diagnostic and/or prognostic relevance or the possibility of such use in the near future.
Genetic Testing
There are a small percentage of individuals with common diseases who have rare, but inherited single gene mutations. These single gene mutations, which increase disease susceptibility, may reach frequencies as high as 1% in some population groups. More common genetic variants (DNA polymorphisms) are also associated with increased risks of developing common conditions. Interestingly, some polymorphisms can actually convey resistance to disease. Nonetheless, other genetic and/or environmental factors must play a role prior to the onset of disease in individuals with either these predisposing rare variants or polymorphisms. Acquired (somatic) mutations have also been found to play a significant role in many human malignancies, including skin tumors. In addition, the functions of genes involved in the development of chronic inflammatory disorders (such as psoriasis and atopic dermatitis) are being elucidated through comparative molecular genetic profiling of cells from diseased organs/tissues with corresponding normal cells. With such knowledge, interventions can be developed which either mitigate disease triggering events or facilitate early and effective therapy.