1.1 Introduction
Acne vulgaris is the result of multifactorial processes in and around the pilosebaceous unit. Currently, the major pathogenic factors are thought to be:
Androgens []
Sebum []
Abnormal keratinization []
Propionibacterium acnes ( P. acnes ) []
Innate immune system-mediated inflammation []
The traditional notion that these factors contribute independently to acne development has been replaced by a more nuanced understanding of their complex interplay. Though the multifactorial nature of acne pathogenesis makes effective treatment challenging, research has shed light on both the mechanisms of action of current treatments and discovered new targets for acne therapy.
1.2 Overview of the Innate Immune System
The immune system consists of both innate (rapid response but no memory to pathogens) and adaptive (delayed, antigen-specific response forming memory) mechanisms against pathogens [].
1.2.1 Toll-Like Receptors
Toll-like receptors (TLRs), a subtype of PRRs found on immune cells (such as neutrophils, monocytes/macrophages, and DCs), serve as potent initiators of innate immune responses. While nearly a dozen TLRs have been identified, TLR2 and TLR4 seem to play the greatest roles in acne pathogenesis (via P. acnes activation) [].
1.2.2 Inflammation via Innate Immune System
Innate immunity-mediated inflammation is both a precipitating and propagating factor in acne pathogenesis. The key player appears to be IL-1. There are multiple hypotheses about what stimuli promote IL-1 production. IL-1 upregulation may be mediated by increased sebum production and breakdown of the skin barrier from decreased linoleic acid in the follicle [].
1.3 Development of Acne Lesions
Acne lesions start as microcomedones, which are present in clinically normal appearing skin and can mature into visible noninflammatory comedones or inflammatory papules. While the multifactorial and synergistic nature of acne pathogenesis has long been recognized, in vitro studies have shed light on the extensive cross talk that occurs among the various factors and the innate immune system. Under physiologic circumstances, the skin harnesses the innate immune response to protect the internal environment from extrinsic pathogens. However, collateral damage to adjacent tissues is an unavoidable consequence of this protective mechanism and manifests clinically as inflammatory conditions such as acne. It has become clear that acne is a disease of the innate immune response, as acne lesions have been shown to express higher levels of inflammatory components compared to normal skin. Studies have revealed that the initial microcomedo is a product of inflammation and hyperproliferation of the follicular epithelium [].
1.3.1 Androgens
Androgens are involved in promoting growth of sebaceous glands and sebum secretion, inducing keratinocyte proliferation, stimulating hair growth, and inhibiting wound healing [].
1.3.2 Sebum
Sebum is secreted by sebaceous glands through holocrine secretion (extrusion of the entire cell). The primary components of sebum include squalene, cholesterol, cholesterol esters, wax esters, and triglycerides [].
Androgens and retinoids regulate sebum production by sebaceous glands. Functional androgen receptors have been identified on the basal layer of sebaceous glands, and androgens stimulate sebaceous gland growth and secretion []. These findings suggest a role of the sebaceous gland in neuroendocrine function and the stress response.
In addition to lubricating skin and hair, sebum also plays a role within the innate immune system through multiple pathways. Increased production of sebum is necessary but not sufficient for acne pathogenesis; the composition of sebum lipids also has an impact on inflammation. There are increased free fatty acids, squalene, and squalene oxidase and decreased linoleic acid, conditions which promote hypercornification of the follicle through direct and indirect modulation of the innate immune system [].
Sebum-mediated acne pathogenesis occurs in concert with P. acnes . The anaerobic environment of sebaceous glands and sebum lipids allows proliferation of P. acnes , which expresses lipases that break down triglycerides into proinflammatory free fatty acids []. This complement of findings suggests the important role of sebaceous glands in pathogen recognition and in regulation of the innate immune system on the skin surface.
1.3.3 Propionibacterium acnes ( P. acnes )
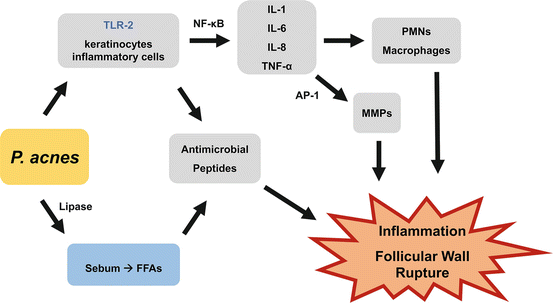
P. acnes is a commensal anaerobic, gram-positive rod whose interaction with the innate immune systems plays a significant role in acne development. This bacteria expresses lipases that convert sebum triglycerides into free fatty acids, which stimulate release of antimicrobial peptides and therefore inflammation and comedogenesis [):
Fig. 1.1
P. acnes promotes acne pathogenesis through multiple pathways involving the innate immune system
TLR-mediated release of cytokines and chemokines (IL-1, IL-6, IL-8, and TNF), which then recruit neutrophils (via IL-8) and macrophages to the pilosebaceous unit and promote inflammation and rupture of the follicular wall. Macrophages propagate the cycle by releasing IL-8 (more neutrophil recruitment) and IL-12 (promotes Th1 response) [].
TLR-mediated release of cytokines and chemokines that also amplifies the AP-1 transcription factor, which induces production of matrix metalloproteinases (MMPs) [].
TLR-mediated expression of antimicrobial peptides (HD1 and 2, cathelicidin, and granulysin), which also contribute to inflammation and follicular wall rupture [].
P. acnes also promotes differentiation of monocytes to macrophages (expressing CD209), which then phagocytize microbes, including P. acnes . ATRA has been shown to promote this same differentiation, which suggests an antimicrobial mechanism of action for ATRA []. This may contribute to the hyperkeratinization seen in comedones.
P. acnes produces biofilm, a polysaccharide lining around a collection of microbes that enhances adherence within the follicle. Biofilm can also increase the stickiness of sebum and impede keratinocyte desquamation, leading to the keratin plug seen in comedones. Biofilm also enhances P. acnes resistance to antibiotics [].
1.3.4 Abnormal Keratinization
The microcomedo is the initial lesion of acne development. Previously, abnormal keratinization was thought to precede inflammation associated with acne. However, it has become clear that IL-1-mediated inflammation precedes hyperkeratinization. In fact, IL-1 has been localized to open comedones [].