Classification: Antidote, adsorbent.
Action: When certain chemicals and toxins are in proximity to the activated charcoal, the chemical will attach to the surface of the charcoal and become trapped.
Indications: Toxic ingestion.
Adverse Effects: Nausea/vomiting, constipation, or diarrhea. If aspirated into the lungs, charcoal can induce a potentially fatal form of pneumonitis.
Contraindications: Ingestion of acids, alkalis, ethanol, methanol, cyanide, ferrous sulfate or other iron salts, lithium; coma; GI obstruction, known minimally toxic ingestion.
Adult: 50 to 100 g/dose.
Pediatric: 1 to 2 g/kg.
Pregnancy class C.
Jones & Bartlett Learning.
Classification: Antiarrhythmic.
Action: Slows the conduction of electrical impulses at the AV node.
Indications: Stable reentry SVT. Does not convert AF, atrial flutter, or VT.
Adverse Effects: Common adverse reactions are generally mild and short-lived: sense of impending doom, complaints of flushing, chest pressure, throat tightness, numbness. Patients will have a brief episode of asystole after administration.
Contraindications: Sick sinus syndrome, second- or third-degree heart block, poison-/drug-induced tachycardia, asthma, or bronchospasm.
Dosage: Note: Adenosine should be delivered only by rapid IV bolus with a peripheral IV or directly into a vein, in a location as close to the heart as possible, preferably in the antecubital fossa. Administration of adenosine must be immediately followed by a saline flush, and then the extremity should be elevated.
Adult: Initial dose 6 mg rapid IV, IO (over a 1- to 3-second period) immediately followed by a 20-mL rapid saline flush. If the first dose does not eliminate the rhythm in 1 to 2 minutes, administer a second dose of 12 mg rapid IV, IO.
Pediatric:
 Children >110 pounds (50 kg) : Same as adult dosing.
Children >110 pounds (50 kg) : Same as adult dosing.
 Children <110 pounds (50 kg) : Initial dose 0.1 mg/kg IV, IO (max dose: 6 mg) immediately followed by a 5-mL rapid saline flush; may repeat at 0.2 mg/kg (max dose: 12 mg).
Children <110 pounds (50 kg) : Initial dose 0.1 mg/kg IV, IO (max dose: 6 mg) immediately followed by a 5-mL rapid saline flush; may repeat at 0.2 mg/kg (max dose: 12 mg).
Special Considerations:
Use with caution in patients with preexisting bronchospasm and those with a history of AF.
Older adults with no history of paroxysmal SVT (PSVT) should be carefully evaluated for dehydration and rapid sinus tachycardia requiring volume fluid replacement rather than simply treated with adenosine.
Pregnancy class C.
ALBUMIN

Classification: Volume expander, colloid.
Action: Increases oncotic pressure in intravascular space.
Indications: Expand intravascular volume.
Adverse Effects: Allergic reaction in some patients; an excessive volume of fluid can result in congestive heart failure and pulmonary edema in susceptible patients.
Contraindications: Severe anemia or cardiac failure in the presence of normal or increased intravascular volume, solution appears turbid or after 4 hours since opening the container, known sensitivity.
Dosage: Two preparations: 500 mL of a 5% solution (250 mL of a 5% solution also available) and 100 mL of a 25% solution (50 mL of a 25% solution also available).
Adult:
 5% albumin: 500 to 1,000 mL IV, intraosseous (IO).
5% albumin: 500 to 1,000 mL IV, intraosseous (IO).
 25% albumin: 50 to 200 mL IV, IO.
25% albumin: 50 to 200 mL IV, IO.
Pediatric:
 5% albumin: 0.5 to 1 g/kg IV; the initial dose may be repeated in 15 to 30 minutes if the clinical response is inadequate.
5% albumin: 0.5 to 1 g/kg IV; the initial dose may be repeated in 15 to 30 minutes if the clinical response is inadequate.
 25% albumin: 1 g/kg IV, IO.
25% albumin: 1 g/kg IV, IO.
Special Considerations:
Patients with a history of congestive heart failure, cardiac disease, hypertension, and pulmonary edema should be given 5% albumin, or the 25% albumin should be diluted. Because 25% of albumin increases intravascular volume greater than the volume administered, slowly administer 25% albumin in normovolemic patients to prevent complications such as pulmonary edema.









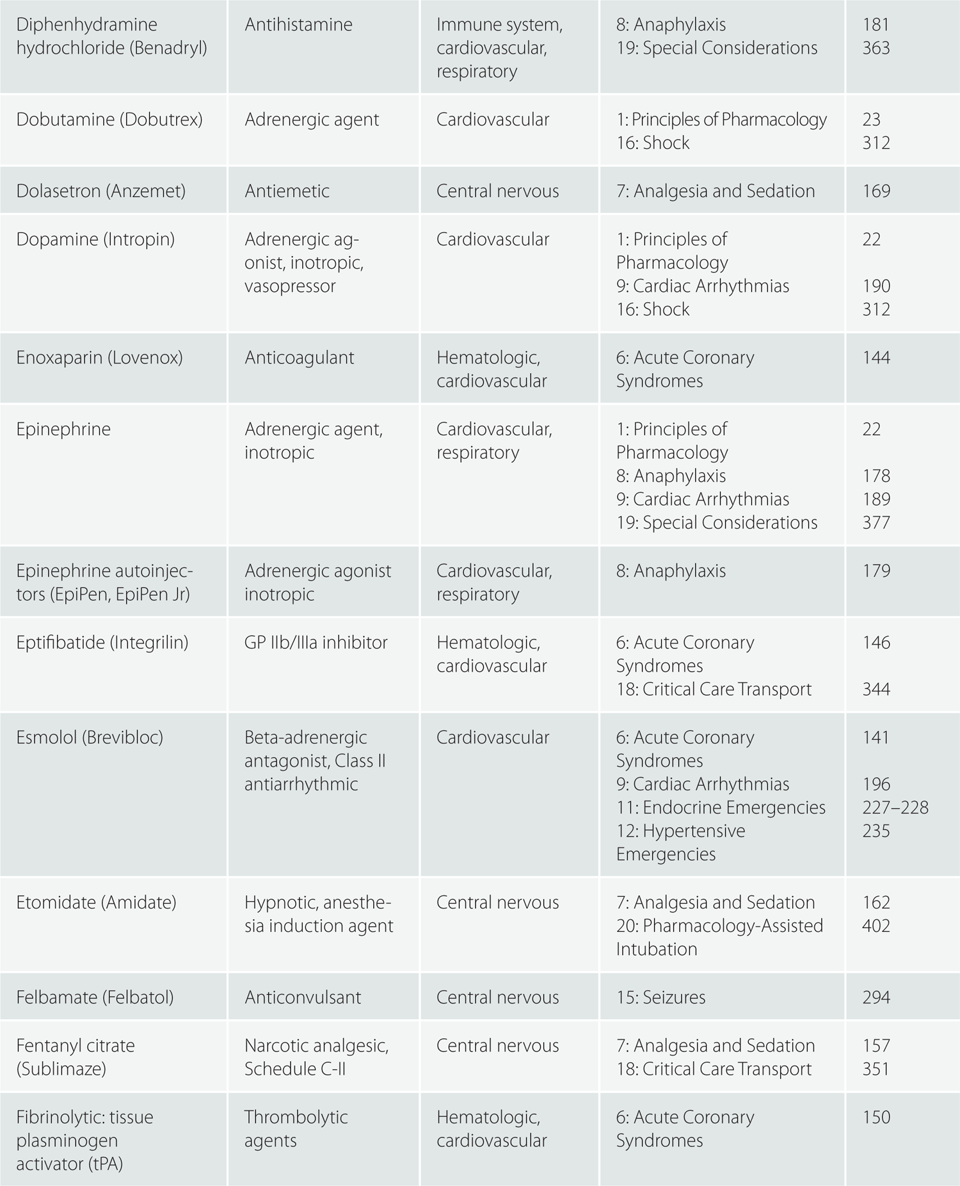
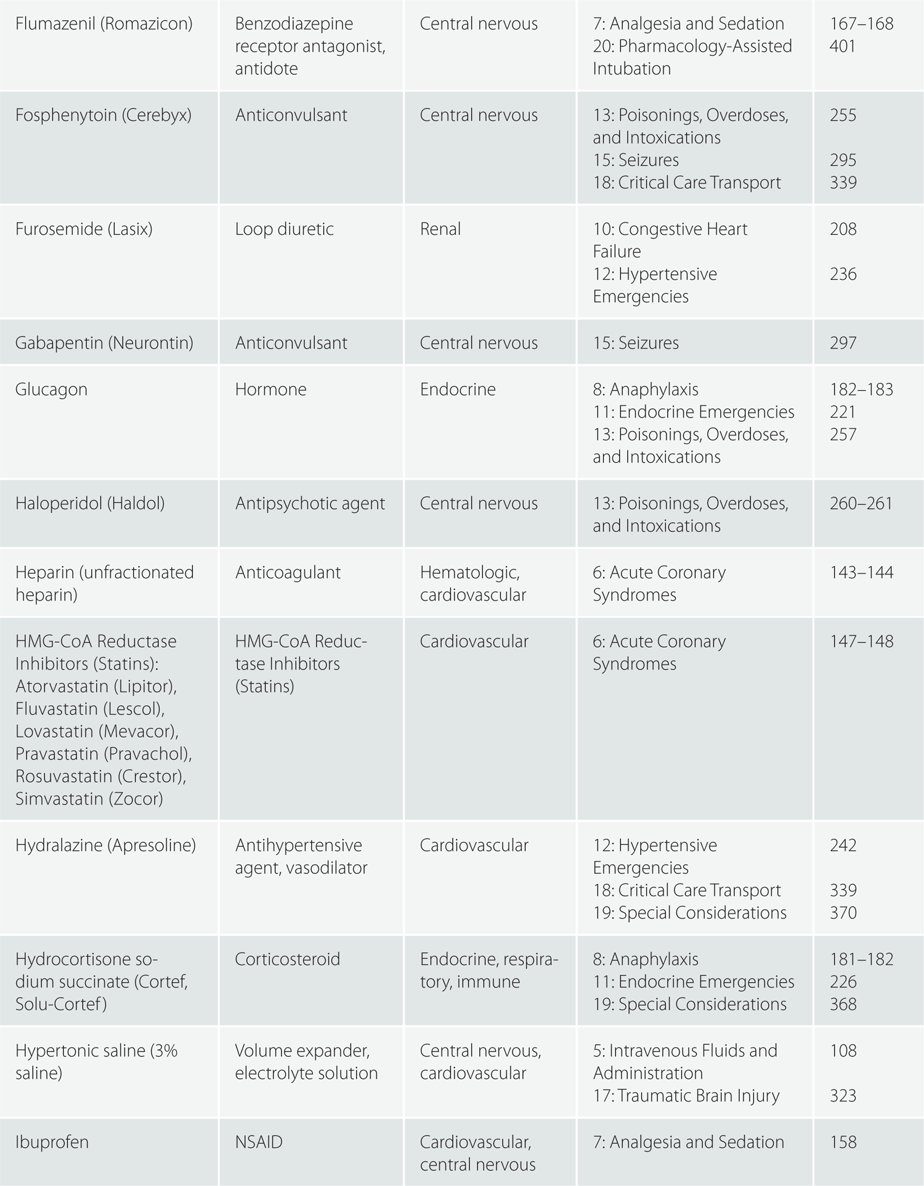
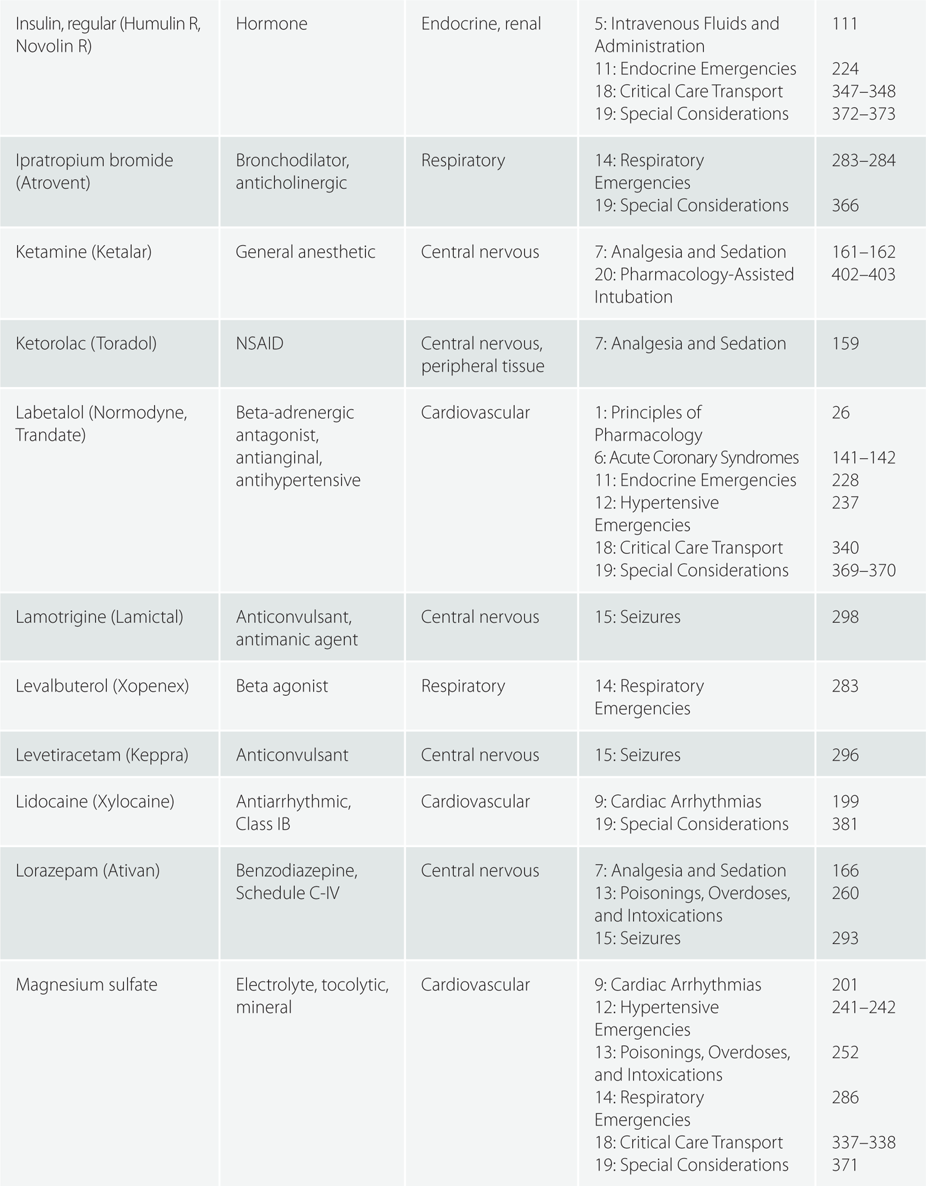
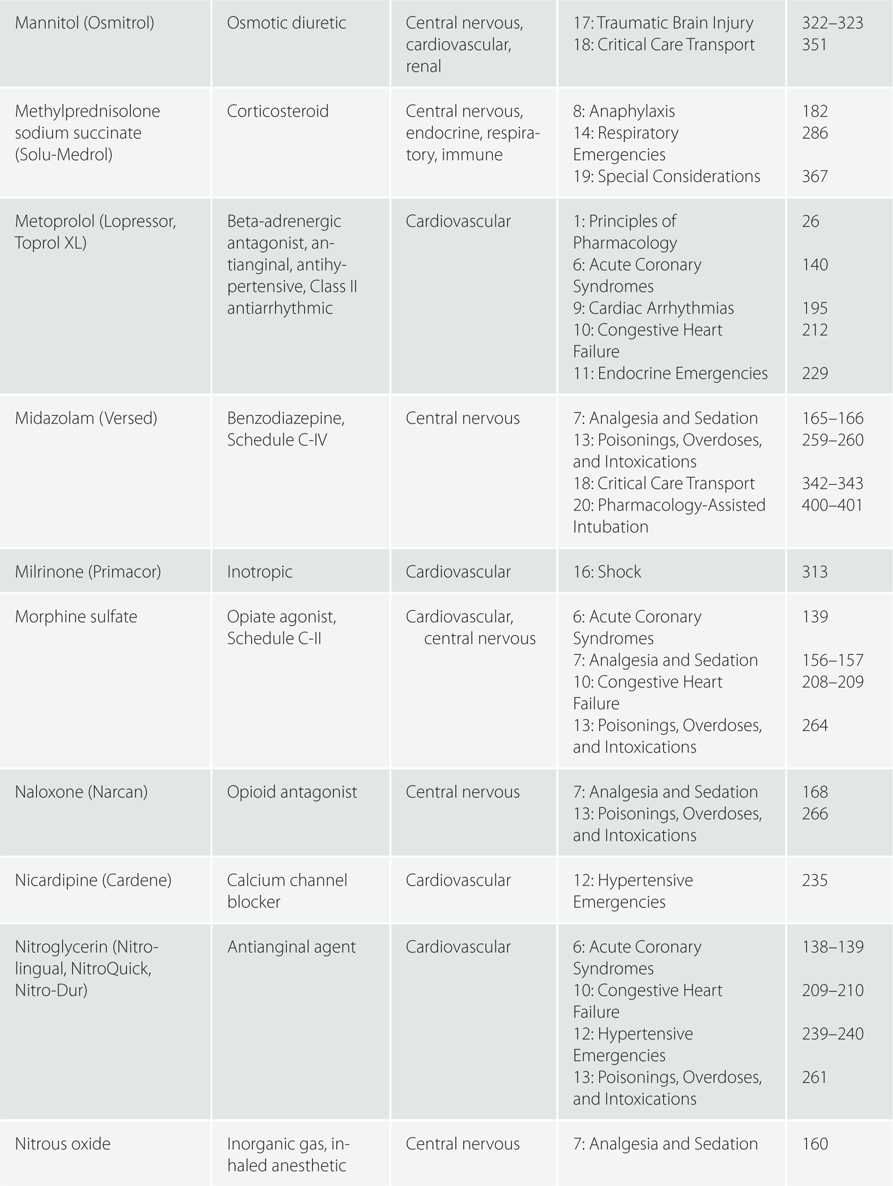


 Children >110 pounds (50 kg) : Same as adult dosing.
Children >110 pounds (50 kg) : Same as adult dosing.